Introduction
Chimeric Antigen Receptor modified T cells – known as CAR T – is a powerful new tool for treating cancer. CAR T therapy starts by extracting a patient’s immune cells from their body. The cells are genetically engineered to recognize a patient’s own tumour, and then returned to the patient’s body in large numbers. This is known as a type of adoptive cell transfer. The re-engineered cells are able to specifically attack and kill the cancer cells. This therapy is having dramatic responses in some patients with certain types of advanced cancer – such as paediatric and adult patients with blood cancers that include acute lymphoblastic leukemia (ALL) and lymphoma.
Because CAR T therapy is very personalized (it requires genetically engineering the patient’s own T cells), there is considerable infrastructure and expertise required to deliver treatment safely and successfully. The genetic modification step involves introducing into the T cells an extra gene using a vector that carries instructions to recognize tumour cells. As a result, the T cell becomes covered with a new component instructed to recognize tumour cells called a Chimeric Antigen Receptor (CAR). It is “chimeric” because it contains different receptor sub-components fused together, and it is an “antigen receptor” because it recognizes specific features, or antigens, on the surface of tumour cells. To make a CAR-T cell, a vector, oftentimes a virus, is used to safely introduce the ‘CAR’ into T cells. Both the vector containing the new tumour recognition receptor, and the engineered CAR T cells are grown in specialized laboratories called clean rooms. Once engineered CAR T cells multiply in the billions in the clean room, they are returned to the clinic to be re-administered to the same patient from whom the T cells were taken.
Due to the considerable infrastructure and expertise to deliver CAR T safely and successfully, there had been very few CAR T clinical trials in Canada. BioCanRx recognized the need to boost infrastructure and manufacturing capacity in Canada to support bench to bedside research and to ultimately increase access to CAR T by increasing the number of clinical trials available to Canadian patients. This new capacity will also pave the way to deliver on Canadian innovations in the engineered T cell space, which right now is not possible to move these innovations beyond the laboratory stage of development.
“Immunotherapies like CAR T therapy work by boosting the body’s immune system, to help it recognize and kill cancer cells. We know now that by taking T cells out of patients and reengineering them, we can get them to have dramatic responses in some kinds of cancer patients. Unfortunately, in Canada, we don’t have this technology available to us to be used widely across the country. So BioCanRx investments are allowing us to be in a position to manufacture this kind of product ourselves, get our own scientists engaged in being able to actually test their ideas, exploiting this new technology and, we hope, to bring something to the Canadian people much faster than it would be otherwise.” – Dr. John Bell Scientific Director, BioCanRx
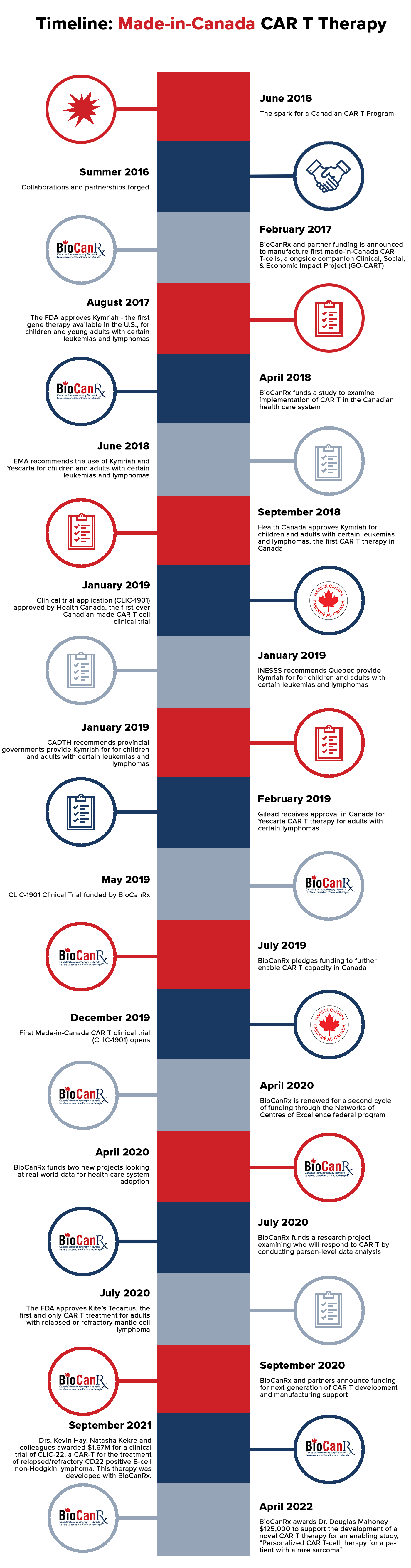
Made-in-Canada CAR T Therapy Timeline
June 2016: The Spark for a Canadian CAR T Program
At the Summit for Cancer Immunotherapy, BioCanRx’s inaugural scientific conference, a group of BioCanRx network investigators were inspired by a session on advances in antibody therapeutics and engineering. They were chatting at a social event when one of them asked – “what if we could treat Canadian patients with CAR T-cell therapy by developing clinical CAR T manufacturing capabilities in Canada?” These would be the first made-in-Canada CAR-T cells. Sharing research ideas at this first conversation were Drs. John Bell (BioCanRx Scientific Director), Harry Atkins and Natasha Kekre (The Ottawa Hospital) and Robert Holt, and Brad Nelson (BC Cancer). Their idea sparked a new collaboration that ultimately would lead to a ‘made-in-Canada’ CAR T-cell program to provide access to this therapy for Canadian patients right here in Canada.
Summer 2016: Collaborations and Partnerships Forged
BioCanRx network investigators in Ottawa, Vancouver and Victoria began working on project proposals to secure funding, partners and expertise to develop the safe and efficient production of CAR T therapy treatment in Canada using a point of care manufacturing approach. The goal was to provide access at this time while also to support the clinical delivery of new CAR T-cell development. Each research team had the necessary ingredients to support a made-in-Canada approach: construct manufacture in Vancouver (Rob Holt), viral vector lentivirus manufacturing in Ottawa and finally, manufacture of the CAR T-cell product in Victoria to produce the product that would eventually be infused into patients. As the final products are to be administered in the clinic, these needed to be manufactured under Good Manufacturing Practices or GMP requiring access to highly specialized equipment and facilities that are part of the Canadian biomanufacturing ecosystem. BioCanRx coordinated meetings and workshops to enable network scientists and clinicians to hammer out the details of how to make all of these different pieces come together, achieving the synergy necessary to manufacture this product. This resulted in applications to BioCanRx to two tandem and synergistic programs: Enabling Studies and Clinical, Social, Economic Impact programs.
February 2017: BioCanRx and partner funding announced to manufacture first made-in Canada CAR T cells
BioCanRx and partners announced funding of almost $5M for research aimed at developing Canada’s clinical CAR T manufacturing capabilities. Canada had the essential infrastructure in place but required a coordinated effort by multi-institutional researchers, and onboarding of CAR T-cell manufacturing capabilities. This funding was necessary to fully develop and technology transfer the expertise and capacity required to deliver this new technology. BioCanRx invested in research projects advancing several innovative engineered T cell designs, which further benefitted from this infrastructure and capacity investment, and accelerated delivery of these novel concepts into clinical testing in Canada. Leading the Enabling Study project Capacity building for Chimeric Antigen Receptor (CAR)-modified T cell therapies in Canada were Drs. Rob Holt, John Bell, Natasha Kekre and John Webb.
Media outlets were interested in this news:
Ottawa poised to become manufacturing centre for cancer-fighting agent – The Ottawa Citizen, Feb 16, 2017
BC researchers Working to bring game changing cancer treatment to Canada – Global News, March 14, 2017
To help ensure CAR T-cell therapy was brought to patients safely and effectively, BioCanRx funded a companion Clinical, Social, and Economic Impact project Getting better Outcomes with Chimeric Antigen Receptor T-cell therapy (GO–CART): A BioCanRx Research Excelerator to Safely and Effectively Translate CAR-T Cell Therapy for Hematological Malignancies. It reviewed the existing base of knowledge and involved patients at project inception to design a rigorous CAR T clinical trial protocol ready to implement once the products were ready for a phase 1 clinical trial. The research team also examined the economic considerations of implementing this kind of technology in the Canadian health care system. Leading this project were Drs. Manoj Lalu and Dean Fergusson, of The Ottawa Hospital, in collaboration with The Leukemia and Lymphoma Society of Canada and two patient partners: Terry Hawrysh and Stu Schwartz.
Dr. Lalu describes the GO-CART project
Read the published GO-CART study.
August 2017: FDA approved Kymriah (tisagenlecleucel) – the first gene therapy available in the United States.
Kymriah was approved for certain paediatric and young adult patients with a form of ALL. Read more here.
April 2018: BioCanRx funded a study to examine various policy models to examine implementation of CAR T in the Canadian health care system.
In the Canadian publicly funded health-care system, the relatively high cost of CAR T may hinder patient access to this type of treatment in the future. Collaborating with CADTH, Canada’s Health Technology Assessment body, and funded in part by BioCanRx, Dr. William Wong (University of Waterloo) used an innovative approach to establish a platform to support the decision- making process regarding reimbursement and implementation of CAR T-cell therapy in the future. For example, Dr. Wong used a health system–level discrete event simulation model to examine the potential impact of increasing wait times on CAR T-cell therapy effectiveness and showed that modest delays in CAR T-cell therapy significantly hinder its effectiveness. Indeed, increasing the wait time of receiving CAR T-cell therapy in patients with relapsed or refractory diffuse large B-cell lymphoma from 1 to 9 months increased the predicted 1-year mortality rate from 36.1% to 76.3%. Results of this research provide an evidence-based evaluation of this therapy and its place in the health system, and serve as a foundation for clinical trial researchers and policy makers for improving oncology care.
Read the published report here.
June 2018: European Medicines Agency has recommended the use of Kymriah and Yescarta.
Kymriah is recommended for the treatment of paediatric and young adult patients (up to 25 years of age) with refractory or relapsed B-cell ALL, and in adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy. Yescarta (Axicabtagene ciloleucel) is indicated for the treatment of adult patients with relapsed or refractory DLBCL and primary mediastinal large B-cell lymphoma, after two or more lines of systemic therapy. Read more here.
September 2018: Health Canada approved Kymriah, the first CAR T-cell therapy approved in Canada for relapsed or refractory pediatric and young adult B-cell ALL and adult relapsed or refractory diffuse large B-cell lymphoma indications. Read more here.
January 2019: Health Canada approved the first-ever Canadian-made CAR-T cell clinical trial application
Within 2 years of the start of the BioCanRx Enabling Study project Capacity building for Chimeric Antigen Receptor (CAR)-modified T cell therapies in Canada, the team, including Drs. Natasha Kekre, Rob Holt, John Bell, and John Webb received Health Canada approval for Canadian-Led Immunotherapies in Cancer: CLIC-1901 for the Treatment of Patients with Relapsed/Refractory CD19 Positive Hematologic Malignancies.
January 2019: INESSS released its decision recommending Quebec provide Kymriah for children and young adults with diffuse large B-cell ALL and patients with relapsed or refractory B-cell lymphoma. Read more here and here.
January 2019: CADTH released its decision recommending provincial governments provide Kymriah for children with refractory or relapsed B-cell ALL and adults with refractory or relapsed diffuse large B-cell lymphoma. Read more here.
February 2019: Gilead received approval in Canada for Yescarta CAR T-cell therapy for adults with relapsed or refractory large B-cell Lymphoma after two or more lines of systemic therapy. Read more here.
May 2019: CLIC-1901 Clinical Trial funded by BioCanRx
Dr. Natasha Kekre leads the clinical trial titled: Canadian-Led Immunotherapies in Cancer: CLIC-1901 for the Treatment of Patients with Relapsed/Refractory CD19 Positive Hematologic Malignancies. This trial uses Chimeric Antigen Receptor modified T cells as a new tool for treating patients who have had poor responses to other treatments. CAR T cells are made by isolating a sample of a patient’s T lymphocytes from their blood, genetically modifying and activating the cells in the lad, and then re-administering them to the same patient, allowing for a patient’s immune cells to be targeted against their tumour. This is the first clinical trial of Canadian-made CAR-T cells.
July 2019: BioCanRx pledges $1.8 M to further enable CAR T capacity in Canada
The BioCanRx CAR T program utilizes closed and automated CAR T cell manufacturing equipment to fully capitalize on the advantages of automation. As part of its proposed ‘KT on CAR T’ program in Cycle 2, BioCanRx pledged to invest greater than $1.8M in knowledge translation activities to enable sites across Canada to manufacture their own CAR T-cells using such closed and automated equipment. By building out this ‘point-of-care’ manufacturing network, we will enable delivery of these life-saving therapies at points closer to where patients are treated while also supporting the development of new CAR Ts that could be used to treat other cancers. In collaboration with regulators and over the next four years, BioCanRx is committed to scaling up a total of four, point-of-care manufacturing hubs across Canada that will manufacture CAR T products for use in clinical trials. Through its dedication to technology development and access to manufacturing, BioCanRx is making targeted investments with a vision of making this novel therapeutic approach more broadly applicable to as many patients as possible.
December 2019: First Made-in-Canada CAR T Clinical Trial Opens
BioCanRx’s made-in-Canada Chimeric Antigen Receptor T (CAR T) research program, opened the first made-in-Canada CAR T clinical trial at The Ottawa Hospital led by Dr. Natasha Kekre and at BC Cancer in partnership with the Vancouver General Hospital led by Dr. Kevin Hay. The trial (Canadian-Led Immunotherapies in Cancer 01 or CLIC-1901), is designed for patients aged 18 to 75, with acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma who are not responding to other therapies. It is the first trial that uses Canadian-made CAR T cells and, if successful, it could lead to even better CAR T therapies that target more types of cancer and more research innovations available to Canadian patients a point of care manufacturing model. The clinical trial is funded by BioCanRx and is also supported by the BC Cancer Foundation, The Ottawa Hospital Foundation and the Ontario Institute for Cancer Research.
“CAR-T therapy is a game-changer for people with certain kinds of blood cancer, but we need more research to make it even better. Our goal with this first trial is to build Canadian expertise and capacity for innovation in the CAR-T field. We hope this leads to even better CAR-T therapies that work for more kinds of cancer, as well as innovative approaches for providing CAR-T therapy in the Canadian system.” – Dr. Natasha Kekre, Principal Investigator of the CLIC-1901 trial, hematologist and associate scientist at The Ottawa Hospital and assistant professor at the University of Ottawa
April 2020: BioCanRx is renewed for a second cycle of funding through the Networks of Centres of Excellence federal program
In this new phase, BioCanRx looks to expand the reach of its made-in-Canada CAR T program both in terms of manufacturing locations and new types of CAR T-cells. The large clinical pipeline under investigation worldwide demonstrates continuing advancement in CAR T programs for both blood cancers and solid tumors. The importance of translating successful CAR T-cell trials to further add to our cancer-fighting armamentarium is clear. However, complexities in the manufacturing process challenge the large-scale deployment of these therapies. That is why many biotech and pharma are investing in automation to assist in the manufacturing process to enable more robust, less complex, closed and automated CAR T-cell manufacturing.
April 2020 – BioCanRx partnered with the Ontario Institute for Cancer Research and other groups to fund and support two new Clinical, Social, and Economic Impact projects using read-world data
Dr. Kelvin Chan of Sunnybrook Research Institute, is the lead investigator for the project titled: Assessing the Real-World Clinical and Economic Outcomes of Emerging Innovative Technologies in Oncology: The Cases of Biosimilars and CAR T-cells
Dr. Chan and colleagues are performing a health technology assessment to evaluate real-world health outcomes and economic impact of CAR T-cell therapy in Ontario. The team aims to understand real-world uptake, budget impact, effectiveness, safety, and cost-effectiveness of CAR T therapy to allow for system planning, resource allocation, and optimal patient care.
Dr. Kednapa Thavorn, of the Ottawa Hospital Research Institute, is the lead investigator for the project titled: Using real-world data and iterative economic evaluation to prioritize resource allocation for care and research in adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia
To better understand the factors contributing to the cost of CAR T-cell therapies, the potential clinical benefits of the treatment, and what the health system can afford, Dr. Thavorn and her team will apply a continuous economic evaluation framework to CAR T-cell therapy for use in adults with relapsed or refractory B-cell ALL. The process will take a multi-stakeholder approach inclusive of patients, clinicians, researchers and healthcare payers. The results of the study will help facilitate realistic cost of CAR T-cell therapy and assist public payers in setting a price of CAR T-cell therapy based on the additional benefit gained and the other associated costs of the treatment.
July 2020: BioCanRx funds a research project examining who will respond to CAR T by conducting person-level data analysis
A major outcome of the systematic review by Drs. Manoj Lalu and Dean Fergusson of published CAR T-cell trials (funded by BioCanRx) demonstrated that the therapy can be extremely effective in some people and not others. The reasons for this difference remain unclear but emerging evidence suggests that specific characteristics about either the people (for example biological sex or age), disease status or of the therapy itself (dose, for example), can lead to differences in efficacy between patients. In this recently funded Clinical, Social and Economic Impact project, Dr. Dean Fergusson (OHRI) and team will use individual patient data from published CAR T clinical trials to evaluate the impact of these characteristics on response rates. At the same time, the project will provide an updated review of all CAR T-cell trials in blood and solid tumor cancer patients. The results of this analysis will help clinicians and decision-makers optimize CAR T-cell therapy for patients and help inform the design of future clinical trials and interventions that may demonstrate safer outcomes and better efficacy.
July 2020: US FDA approves Kite’s Tecartus (brexucabtagene autoleucel, formerly KTE-X19) the first and only CAR T treatment for adult patients with relapsed or refractory mantle cell lymphoma (MCL). Read more here.
September 2020: BioCanRx announces funding for next generation of CAR T development and manufacturing support
In September 2020, BioCanRx announced funding of $900,000 for two projects to develop the next generation of CAR T-cell therapies. The projects, led by Drs. Kevin Hay (BC Cancer) and Scott McComb (uOttawa/NRC) are titled, “Enabling a Phase I/II Multicenter Clinical Trial of a Novel Single Domain (sd)CD-22-specific Camelid-derived Chimeric Antigen Receptor (CAR) T-cell Therapy” and “Clinical Trial Enabling Studies for Multi-targeted Chimeric Antigen Receptor Therapeutics for the Treatment of B-Cell Malignancies”, respectively.
Additionally, BioCanRx announced significant, targeted investments in domestic point-of-care cell manufacturing and virus biomanufacturing that was first pledged in July 2019. Combined, this almost $3.4M investment is focused on enabling the translation of cutting-edge biotherapeutics through expanding biomanufacturing capacity in Canada for the benefit of Canadian cancer patients.
September 2021
Drs. Kevin Hay (BC Cancer), Natasha Kekre (OHRI) and colleagues are awarded $1,667,700 by CIHR to run a phase I trial of CLIC-2201 – a CD22 CAR-T cell product for the treatment of relapsed/refractory CD22 positive B-cell non-Hodgkin lymphoma. This therapy was developed with BioCanRx Enabling funding in September 2020.
Learn more here
Enabling Project dashboard
April 2022
BioCanRx awards Dr. Douglas Mahoney (University of Calgary) $125,000 in funding to support the development of a novel CAR T therapy in an enabling study titled, “Personalized CAR T-cell therapy for a patient with a rare sarcoma”