Project summary: Enabling Study
Enabling a Phase I/II Multicenter Clinical Trial of a Novel Single Domain (sd)CD-22-specific Camelid-derived Chimeric Antigen Receptor (CAR) T-cell Therapy
April 1, 2020 – March 31, 2023
HIGHLIGHTS

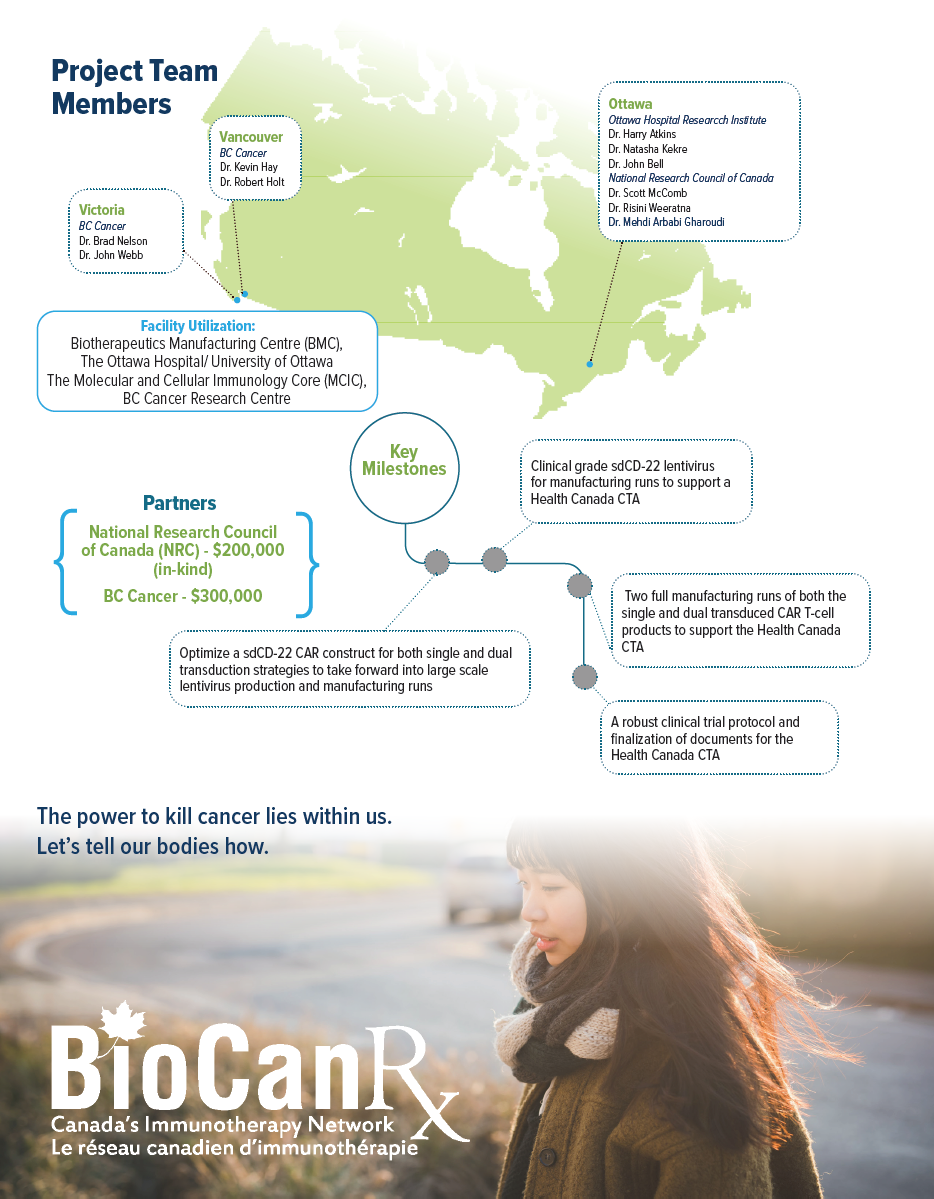
- As part of a previously successful project, BioCanRx invested significant resources in establishing a strong network of investigators and facilities in Canada to develop clinical grade CAR T manufacturing capabilities, with a CD-19 CAR T-cell trial supported by this infrastructure currently enrolling patients (CLIC-01, NCT03765177).
- With a CAR T network in place, BioCanRx investigators in a partnership with the National Research Council of Canada have been developing a complete “antigen to clinic” pipeline, of which this CD-22 enabling study will be the first deliverable.
About the Project
Chimeric antigen receptor (CAR) T-cell therapy targeting CD-19, a marker on B-cell cancers, is a novel approach that has induced meaningful and durable remissions for many patients and is now considered standard of care therapy in relapsed disease. This therapy is manufactured by isolating cells of the immune system called T-cells from a patient, inserting the ‘CAR’ gene into the t-cell using genetic techniques, growing the cells in a clinical laboratory, and then infusing back into the patient. However, despite the success of CD-19 CAR T-cells, only 40-50% of patients have a good long-term outcome.
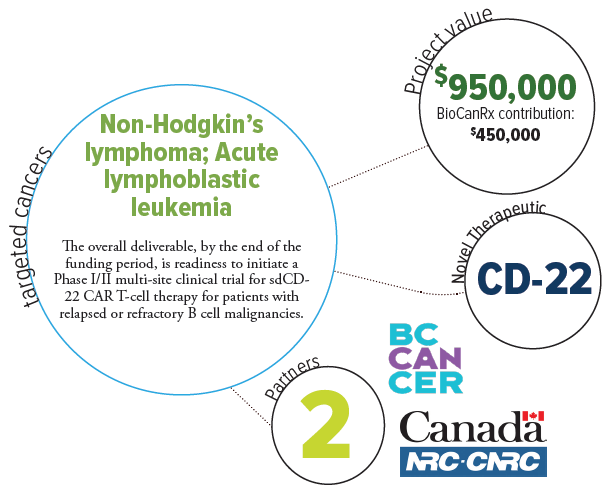
As part of a previous BioCanRx award, the researchers developed domestic capacity for CAR T-cells as a public health effort and launched the first clinical trial of made-in-Canada CD-19 CAR T-cells. They will use this infrastructure and build on this expertise to take another CAR T-cell design, targeting a different B cell marker, CD-22, to clinical trial in order provide (a) dual CD-19 and CD-22 targeting CAR T-cells for leukemia and lymphoma patients as a way of preventing relapse, and (b) CD-22 CAR T-cells for patients with CD-19 negative leukemia and lymphoma.
NRC investigators will develop specific antigen binders for use in CAR T-cell therapy through a high throughput screening assay, and BioCanRx investigators will transfer the binder into a CAR T-cell product ready for the trial through development of clinical grade plasmids, lentivirus, and t-cell products using the BioCanRx infrastructure.
Lead candidate antigen binders for CD-22, derived from camelid single domain antibodies have already been identified by NRC and are ready for input into the BioCanRx network. Enabling the clinical grade development these single domain (sd)CD-22 binders in the BioCanRx network will: (a) demonstrate the ability of BioCanRx investigators to rapidly translate new binders into the clinic, (b) build the network to form a complete pipeline from antigen to clinic, and (c) given the emerging need for improvements to CD-19 CAR T-cells, provide a much-needed therapy for patients with B cell malignancies.