RESEARCH
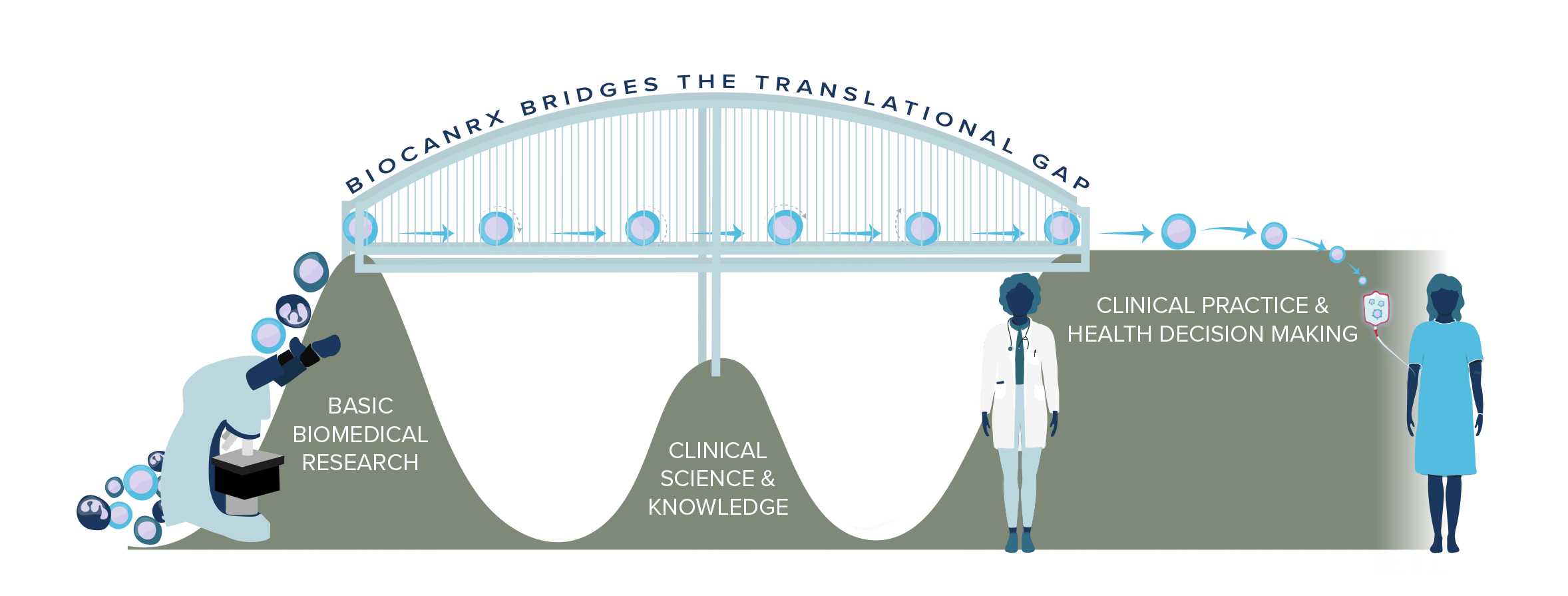
Bridging the Gap
Since its inception in 2015, BioCanRx has built a highly performing translational engine for cancer immunotherapy.
From Lab to Life
Turning Research into Treatments
The network bridges the gap between promising early-stage technologies and their clinical evaluation by taking a comprehensive, multi-disciplinary, ecosystem approach.
Canada has a strong record of health discovery and invention but does not perform as well as it could at translating these discoveries to innovations and treatments. Along the translational path, there are several roadblocks thwarting cancer researchers.

The Challenge
- Fewer curative therapies are reaching Canadian patients through clinical trials.
- Structural barriers slow down the path from lab discovery to bedside treatment.
Our Solution
- BioCanRx removes these barriers with an integrated approach to support new therapeutic discoveries.
- By building on Canada’s existing life sciences investments, we accelerate the benefits of research to patients.
Made-in-Canada Innovation
- Addressing structural issues ensures Canadian discoveries stay in Canada for development.
- Trials are designed to collect real-world data, making future adoption into the health care system more effective.

Kuldeep Neote
Entrepreneur in Residence, FACIT and US National Institutes of Health; Past VP, External Innovation
International Patient Engagement Award
BioCanRx Funded Clinical Trials
Innovative Spinout Companies Launched
Secured Through Partner Funding
Core & Biomanufacturing Facilities Supported
Strategic Partnerships Established Across Sectors
Novel Therapeutic Technologies Developed
Highly Qualified Personnel (HQP) Trained
From Lab to Clinic
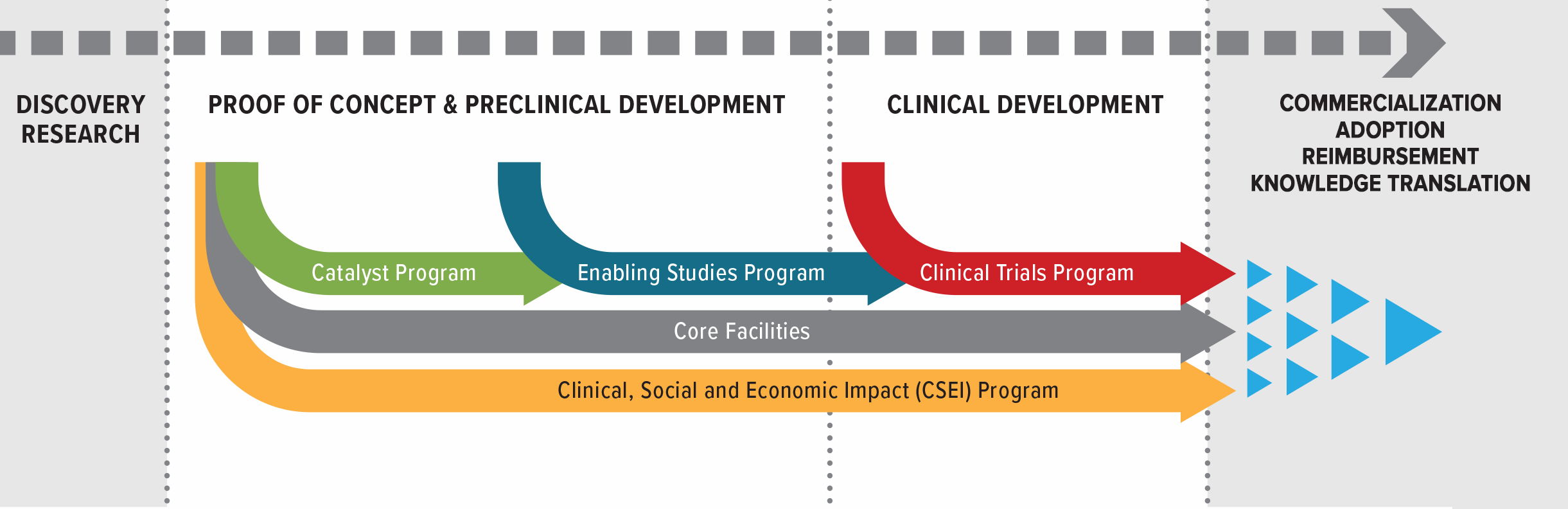
BioCanRx’s Pipeline Approach
BioCanRx’s pipeline approach provides funding to innovations across the translational continuum – from the lab to the clinic, including proof of concept, pre-clinical development, process development and manufacturing, and clinical trials. These projects are supported by our Clinical, Social and Economic Impact (CSEI) program and our Core Facilities. The CSEI program addresses social, legal, ethical, economic, or health-system barriers — such as early health technology assessment (HTA) — as projects progress from pre-clinical research to clinical trials and adoption in the health care system. Core Facilities provide specialized expertise and services that help advance translational research.
Pipeline Funding
Supports all stages from proof of concept to clinical trials.
CSEI Program
Tackles social, legal, ethical, economic, and health-system barriers. (Icon: scales of justice or puzzle pieces)
Core Facilities
Deliver unique expertise and infrastructure for project teams. (Icon: lab building or gear network)
Innovation Journey
BioCanRx Projects Through Different Research Stages
BioCanRx has funded several technologies transitioning from one stage of development to the next via its research funding pipeline. For example, Dr. Robert Holt (BCCA)’s KRAS-targeting cell therapy productwas previously funded as a Catalyst Project and is now funded by an Enabling Studies award to position their innovation for clinical testing. The CLIC-19 CAR T cell therapy has also progressed through BioCanRx’s pipeline from Enabling Study project to Clinical Trial, which has further been supported by the integrated knowledge translation CSEI project, GO-CART.

peptide-MHC antibodies to
target KRAS hotspot mutations
in pancreatic cancer Recombinant TCRs to target
KRAS hotspot mutations in
pancreatic cancer Capacity Building for Chimeric
Antigen Receptor (CAR)-modified T cell
therapies in Canada CLIC-1901 for the Treatment of Patients with
Relapsed/Refractory CD19 Positive
Hematologic Malignancies Getting better Outcomes with Chimeric Antigen Receptor T-cell therapy (GO-CART): A BioCanRx Research Excelerator to Safely and Effectively Translate CAR T-Cell Therapy for Hematological Malignancies

Get Involved
Translational Training Program Fit-for-Purpose
The BioCanRx Ecosystem
More Than the Sum of its Parts

Project intake and performance monitoring
Cancer Stakeholder Alliance & Joint Action Plan, The Learning Institute, Patient-Researcher Roundtable, BioCanRx public forums, online initiatives
Multiple spin-out companies and IP generated
New curative therapies available to patients
Better project outcomes
Engagement of multi-sectoral stakeholders (regulators, patient groups etc.); Funded projects that address clinical, social, and economic impact
Job-ready HQP
Viral Ventures for vector and vaccine development and GMP manufacturing; Addressing the skills gap in GMP though integrative learning program; Initiation of Point of Care network for cellular therapies against cancer