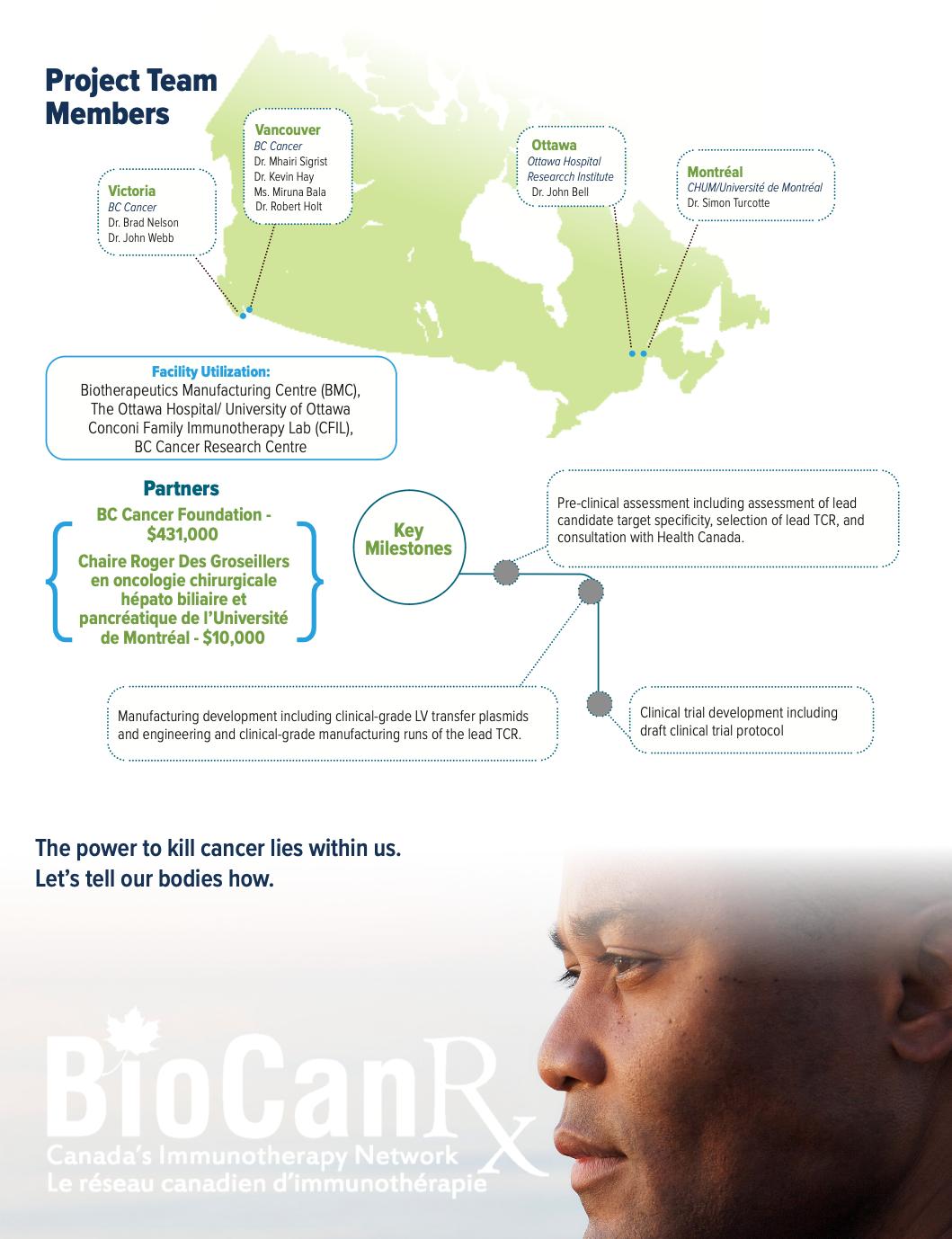
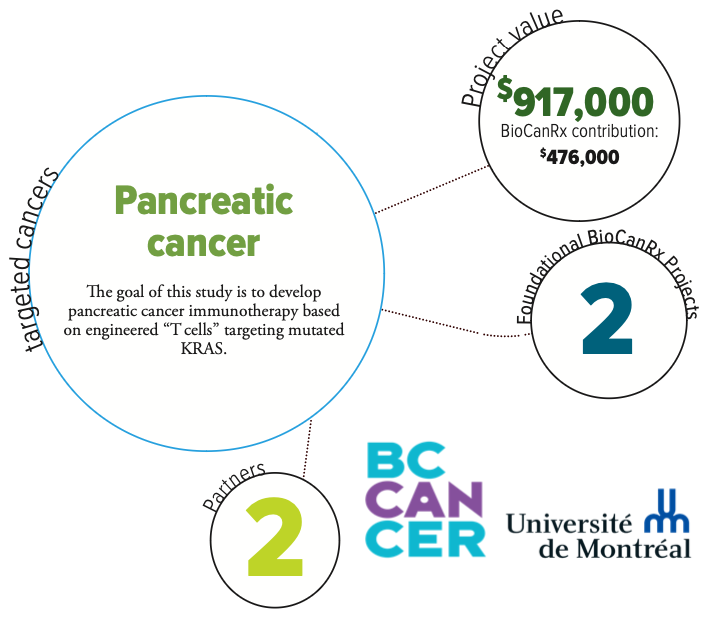
Project summary: Enabling Study
Recombinant TCRs to Target KRAS Hotspot Mutations in Pancreatic Cancer
October 1, 2020 to August 31, 2023
HIGHLIGHTS

- Pancreatic cancer is the fourth leading cause of cancer death among Canadians.
- Nearly all pancreatic cancers are driven by common mutations in a gene called KRAS. Although researchers have not found ways to target mutated KRAS with cancer drugs, recent breakthroughs demonstrate that cancer immunotherapy can be highly effective by targeting mutated gene products.
- KRASG12x is the most common cancer hotspot mutation and A*02:01 is a prevalent HLA allele, so a large patient population stands to benefit, and extends beyond PDAC to other cancer types with these common mutations such as lung and colorectal adenocarcinoma.
About the Project
Recent clinical research has shown that it is possible to engineer an effective T-cell response in patients unable to initiate/sustain anti-tumour immunity. Briefly, t-cells are obtained from blood, genetically modified, activated, and expanded in the lab, and then re-administered to the same patient. The genetic modification step introduces into the T-cells a gene that carries instructions for a new cell surface receptor (previously identified by researchers) that gives the engineered T-cells the ability to recognize and kill cancer cells that have mutated KRAS.
KRASG12D/V hotspot mutations are found in 90% of pancreatic ductal adenocarcinoma (PDAC), but have been undruggable due to the inherent nature of the mutation that locks KRAS in an active state. Mutant KRAS may, however, represent a significant opportunity for cancer immunotherapy. Recent data suggest that peptides bearing KRASG12D/V mutations are naturally presented at the surface of PDAC cells and recognized by functional human T-cells.
This study extends the BioCanRx catalyst project which isolated a panel of TCRs specific to KRAS codon 12 mutational epitopes presented by HLA-A*02:01. Building on the groundwork laid to launch BioCanRx-funded CLIC-01 clinical trial, this enabling study project deliverable is to initiate an anti-KRASG12D/V TCR-engineered T cell adoptive immunotherapy Phase I trial in HLA-A*02:01 patients with metastatic KRASG12D/V PDAC. This project will lay the groundwork for potential benefit – if it is eventually shown to be safe, feasible and efficacious – to Canadian cancer patients.