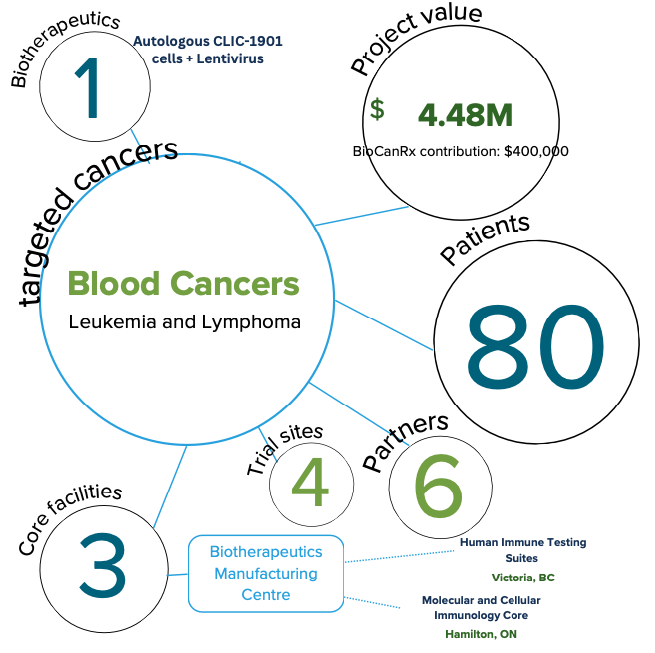
Project summary: Clinical Trial Program
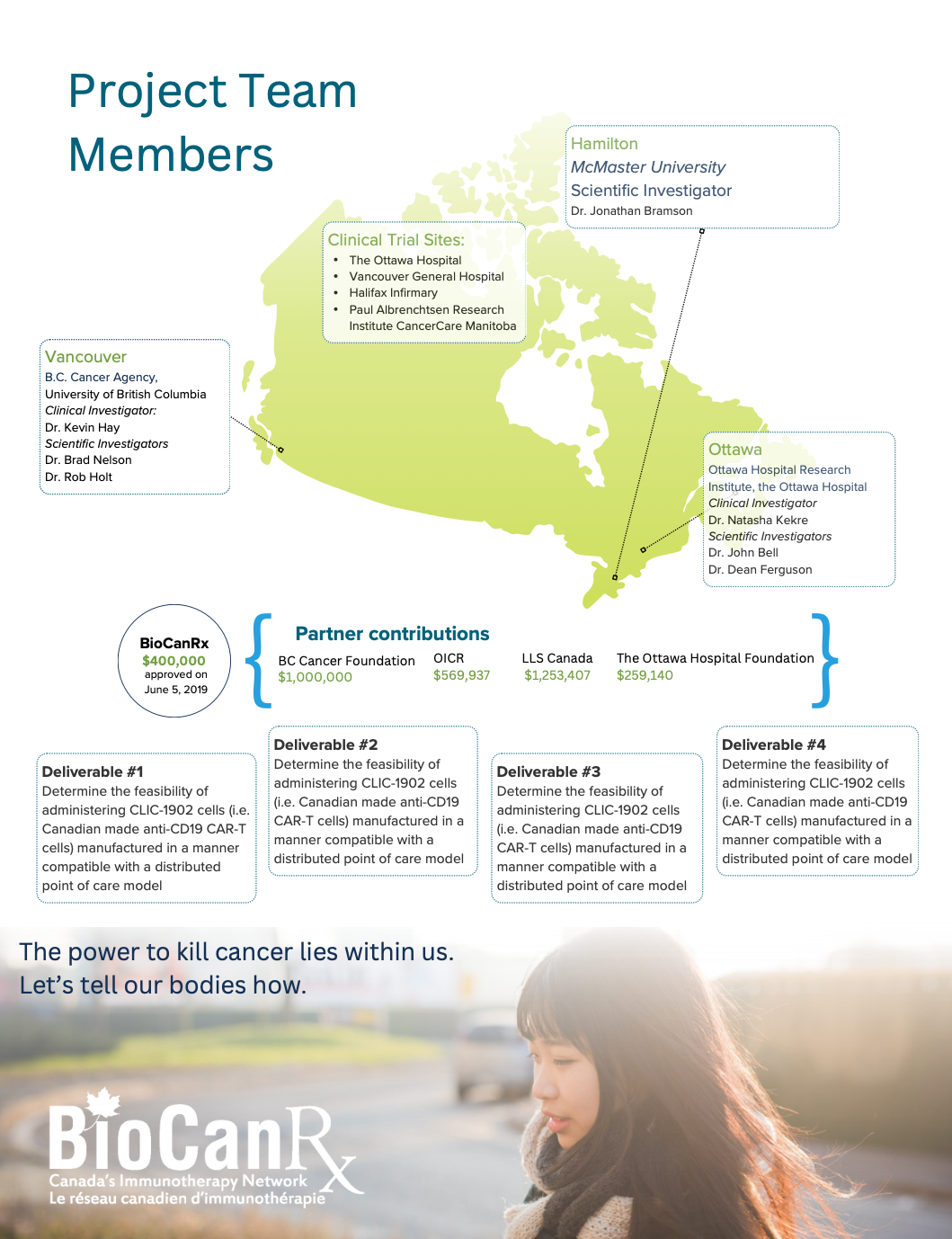
Canadian-Led Immunotherapies in Cancer: CLIC-1901 for the Treatment of Patients with Relapsed/Refractory CD-19 Positive Hematologic Malignancies
Mar 15, 2019 to March 31, 2024
HIGHLIGHTS

- This trial uses Chimeric Antigen Receptor modified T cells as a new tool for treating patients who have had poor responses to other treatments.
- CAR T cells are made by isolating a sample of a patient’s T lymphocytes from their blood, genetically modifying and activating the cells in the lab, and then re-administering them to the same patient, allowing for a patient’s immune cells to be targeted against their tumour.
- CAR T is very personalized, so major infrastructure and expertise are required to produce and deliver this treatment safely and successfully.
- This is the first clinical trial of Canadian-made CAR T cells.
About this project
Patients with some forms of blood cancer that do not respond to standard therapies have a particularly poor chance of survival. Chimeric Antigen Receptor modified T cells (CAR Ts) are a powerful new tool for treating these patients. CAR T cells are made by isolating a sample of a patient’s T lymphocytes (a type of white blood cell) from their blood, genetically modifying and activating the cells in the lab, then re-administering them to the same patient, allowing for a patient’s immune cells to be targeted against their tumour.
The evidence to date for the use of CAR T cells in certain leukemias and lymphomas has been phenomenal in some cases, with durable responses, suggesting that these patients who respond are cured of their underlying malignancy. Because CAR T therapy is very personalized (it requires genetically engineering the patient’s own cells), these is considerable infrastructure and expertise required to manufacture and deliver this treatment safely and successfully.
BioCanRx was integral to providing the funding needed to develop the processes and manufacture the materials needed for CAR T-cell production, and to write the clinical trial application for this to be an approved procedure under Health Canada for a clinical trial. Our team is now ready to provide this therapy and seeks funding from BioCanRx to support the roll-out of this clinical trial, which would fund manufacturing and clinical care costs of patients in the first clinical trial of Canadian-made CAR 1 cells.