BioCanRx Open Call for Research Proposals
Applications Now Closed
Download the Overview and Guidelines
Information Session July 29, 2024
Watch the session
Download the presentation
BioCanRx, Canada’s Immunotherapy Network, is pleased to announce the launch of its first Open Call for Research Proposals made possible by funding from the Strategic Science Fund. Through this call for proposals, BioCanRx will support multi-disciplinary cancer immunotherapy and biotherapeutic approaches.
About BioCanRx
BioCanRx is Canada’s Immunotherapy Network. Our vision is to turn all cancers into curable diseases. We are a network of scientists, clinicians, cancer stakeholders, academic institutions, NGOs and industry partners working together to accelerate the development of leading-edge immune oncology therapies for the benefit of patients. As a leader in the translation, manufacture and adoption of cancer immunotherapies, we invest in translating world-class technologies from the research lab into clinical trials and beyond. BioCanRx provides researchers with access to funding, expertise, training and core facilities. We train and develop the talent needed for a thriving Canadian health biotechnology sector. BioCanRx receives funding from the federal government’s Strategic Science Fund. The network is hosted by the Ottawa Hospital Research Institute.
To date, we represent a network of over 319 scientists and clinicians, 52 NGOs and 40 industry partners working together to accelerate to the clinic the most promising immune oncology biotherapeutics designed to save lives and enable a better quality of life. Since 2015, we have leveraged $29.8M of a $40M investment by the Government of Canada into $116.26M of partner funding in support of excellent research and clinical trials benchmarked to leading international efforts, providing access to novel treatments for cancer patients right here in Canada. This has been rapidly accomplished by linking together critical core facility infrastructure and providing network investigators and partner companies seamless access to the tools they need: expert biotherapeutic pre-clinical to clinical translation advice, specialized training for scientists, trainees and clinicians in biotherapeutic translation, priority access to highly skilled GMP manufacturing and GLP immunoassays, innovative clinical trial design and tailored support for patient engagement. In this way, BioCanRx takes a “whole-of-ecosystem” knowledge translation approach to successfully advance promising biotechnologies in Canada. As a result of this multi-pronged approach, we have rapidly built a compelling portfolio of projects in our project pipeline that is delivering highly innovative clinical trials for, and informed by, cancer patients across Canada.
About BioCanRx’s Research Program
Traditional treatments like surgery, radiation, and chemotherapy have been mainstays of cancer care and have yielded improved outcomes over decades. Yet, they have often fallen short of a cure, and impair cancer patients’ quality of life. This gap underscores the need for innovative approaches in Canada’s cancer strategy.
Immunotherapy and biotherapeutic innovations present a transformative opportunity to shift the battle against cancer. Despite their promise, these approaches often lack the necessary resources for translational research activities and adjacent clinical, social, ethical, and regulatory pathways, policies and innovations to move them from bench to bedside.
BioCanRx takes a translational approach to advance cancer biotherapeutics by funding early-stage proof-of-concept studies, supporting the preparation of products for clinical testing, and conducting novel Phase I/II clinical trials. The program also seeks to identify and address barriers to the adoption of these biotherapeutics, thereby accelerating their translation from research to clinical practice.
About the Open Call for Research Proposals – Cycle 3 Call 1
This Open Call for Research Proposals will use BioCanRx’s established translational research pipeline approach to fund projects aiming to advance promising cancer biotherapeutics and immunotherapies, and support the adoption of these biotherapeutics into the Canadian healthcare systems.
To inform this (and future) Calls for Proposals, this past spring we undertook consultations with the Canadian community of researchers, patient partners and individuals with lived experience, cancer charities and advocacy groups, industry partners, and other stakeholders. We also engaged our Research Management Committee, Board of Directors, and Cancer Stakeholder Alliance. These consultations encouraged BioCanRx keep the research scope and breadth of eligible projects broad to ensure we are investing in the most promising innovations.
Call Objectives & Research Scope
Building on this feedback, we are pleased to invite research proposals spanning various topics falling within cancer immunotherapy and biotherapeutic translation. For the purposes of this call, cancer immunotherapy is defined as all therapeutic products that modulate the immune system.
Proposals should aim to address at least one of the following objectives:
- Accelerate Cancer Immunotherapy and Biotherapeutic Product Innovations from Bench to Bedside: Develop and translate promising innovations from proof of concept through to clinical application (i.e. Phase I/II clinical trials).
- Address the Clinical, Social, Economic, Regulatory and Policy Elements of these Innovations: Identify and address the barriers to the adoption and integration of these therapies into clinical practice and Canada’s healthcare system.
- Support Technology Innovation through Core Facility Activities: Canadian academic infrastructure and technologies that support the advancement and production of cancer immunotherapies and biotherapeutics.
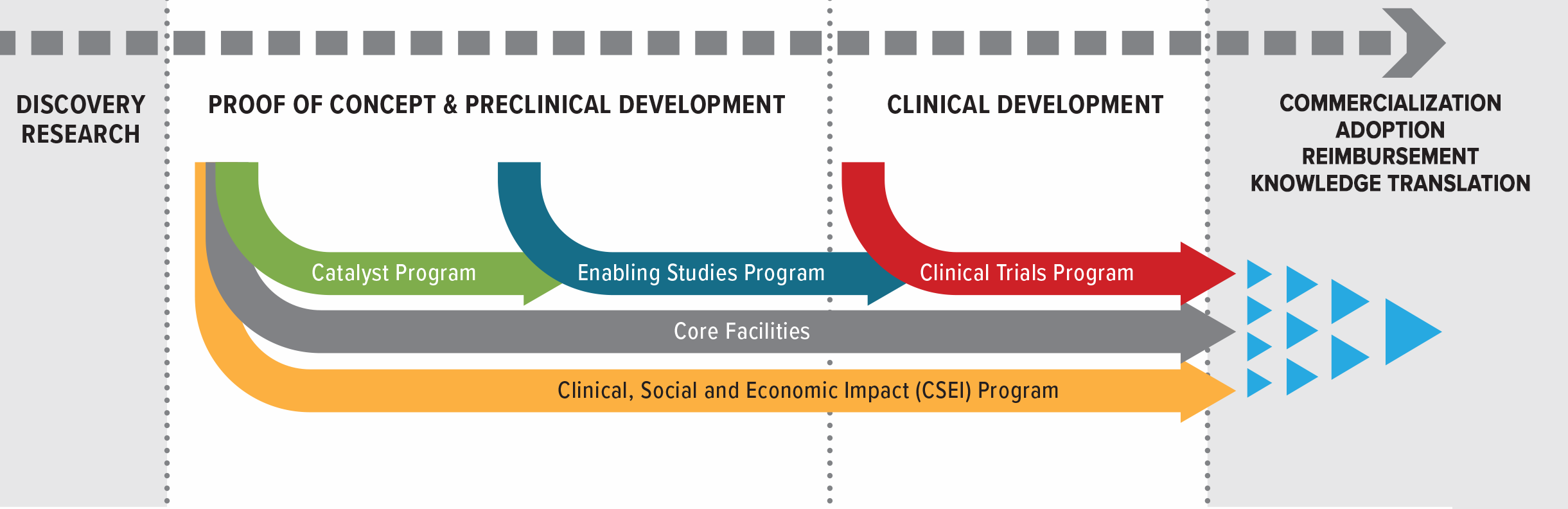
This call for research proposals includes BioCanRx’s five established funding programs:
- Catalyst Program: Short-term, early-stage proof-of-concept studies that will advance cancer biotherapeutics development, assessment or clinical translation. Projects may be oriented toward companion technologies or methodologies, or combination therapeutic strategies.
- Enabling Studies Program: Prepare and position biotherapeutic products and platforms for clinical testing in patients, including GMP manufacturing and process development. Enabling Study projects should result in either CTA submission packages or Quality (Chemistry and Manufacturing) packages.
- Clinical Trials Program: Phase I/II clinical trials of novel cancer biotherapies that have been substantially developed in Canada.
- Clinical, Social and Economic Impact (CSEI) Program: Identify and address the barriers to the adoption and integration of these therapies into clinical practice and Canada’s healthcare system.
- Core Facilities Program: Manufacturing or research technologies and/or services that support the advancement and production of cancer immunotherapies and biotherapeutics.
More information about funding program details, specific project eligibility criteria and the application process and are defined in the Overview and Guidelines document linked above.
Stages of the Call for Proposals
- Notice of Intent (NOI). The NOI is for administrative purposes only. Please send a brief email with the Subject: Open Call – Notice of Intent noting your intention to apply to applications@biocanrx.com. Please indicate the following: 1) project lead, 2) funding program for which you will be applying (Catalyst, Enabling Studies, Clinical Trial, CSEI), 3) draft project title, and 4) 3-5 keywords. A NOI must be submitted prior to gaining access to the LOI form.
The deadline for submission of the NOI is the same as the deadline for LOI submission, however, applicants are encouraged to submit their NOI as early as possible to assist with planning and to ensure sufficient time to complete the LOI by the deadline. - Letter of Intent (LOI). The LOI stage is your opportunity to assemble a multi-disciplinary team and propose a research project to BioCanRx’s Research Management Committee (RMC). The RMC will evaluate LOIs based on their alignment with BioCanRx’s research mandate and eligibility within one of the established funding programs. This call is open to all investigators located at Canadian institutions eligible to receive peer-reviewed funding from the federal research-granting councils (CIHR, SSHRC, NSERC).
- Invitation to the Full Application. Following review by BioCanRx’s Research Management Committee, successful LOIs will be invited to submit a Full Application and will receive further instructions on how to apply.
- Full Application Submission. Full application submissions will also be reviewed by BioCanRx’s Research Management Committee.
Key Dates
- Call launch: July 15, 2024
- Information Session: July 29, 2024
- Notice of Intent (NOI) to apply: prior to LOI submission
- Letter of Intent (LOI) submission deadline: August 26, 2024
- Notification of LOI results and invitation to Full Application: week of October 14, 2024
- Full application submission deadline: December 17, 2024
- Funding award start date: early March, 2025
- Public announcement of funding results: early spring 2025
Information Session:
BioCanRx will be hosting an information session about this Open Call for Research Proposals on July 29, 2-3pm ET. You can register here.
This session will be recorded and made available on this website following the event.
Contact Information
Please direct questions about the program and application process to Julie Jonkhans, Manager of Training and Research Programs (jjonkhans@biocanrx.com) or Stéphanie Michaud, BioCanRx President and Chief Executive Officer) (smichaud@biocanrx.com).

