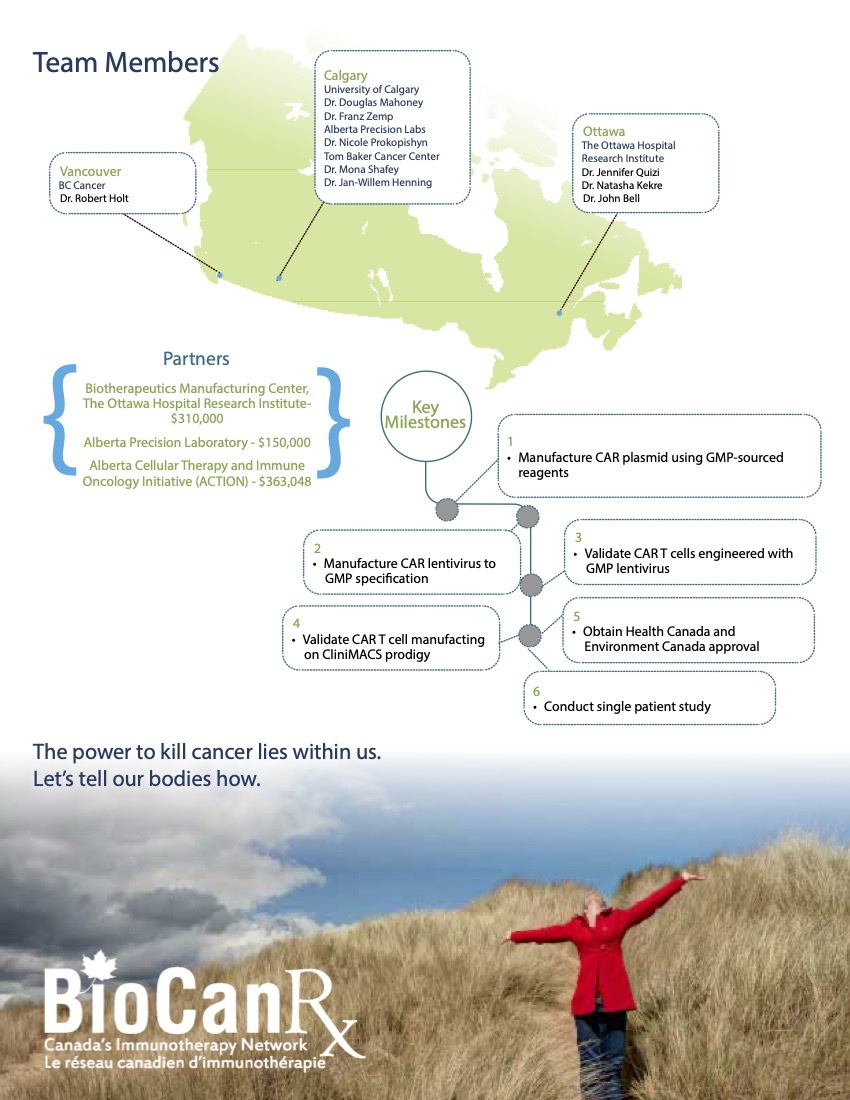
Project summary: Enabling Study
Personalized CAR T-cell therapy for a patient with a rare sarcoma
April 1 2022 – March 31 2023
HIGHLIGHTS

- There are currently no curative treatments for ASPS (a rare orphan cancer)
- The team is underway with developing a novel solid-tumour targeting CAR T therapy for use in a single-patient clinical trial.
- If successful, the study will provide a blueprint and rationale for developing personalized CAR T therapies for other rare orphan cancers.
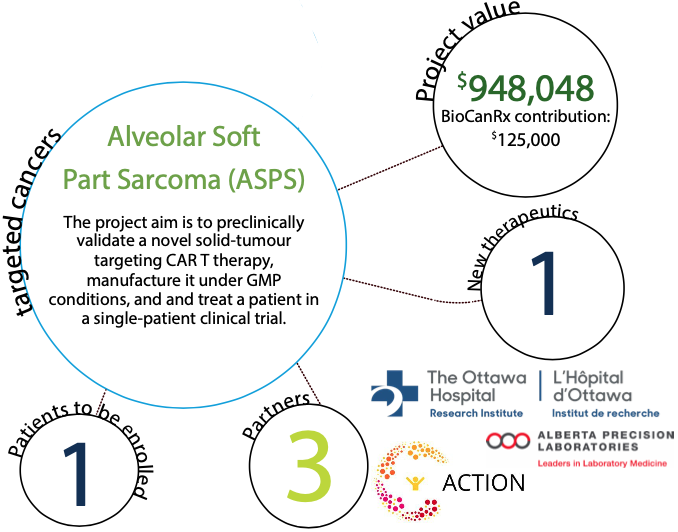
About the Project
In 2015, a 14-year-old female was diagnosed at the Alberta Children’s Hospital pediatric oncology clinic with a rare high-fatality cancer called Alveolar Soft Part Sarcoma (ASPS). There are no curative treatments for ASPS. Over the past 6 years, our patient has undergone 10 surgeries to remove tumour from her leg, lungs, brain, spine, colon, and pancreas. While she is currently in a period of disease quiescence, relapse is deemed inevitable, and our patient will likely run out of surgical options soon.
To address the patient’s unmet clinical need, the research team has developed a personalized therapy by engineering a novel Chimeric Antigen Receptor (CAR) that teaches her immune system to attack and kill her cancer. Over the past year, the team has tested the therapy against her cancer cells grown in the lab and her tumours grown in mice. The treatment was safe and efficiently killed her cancer cells and shrank her tumours in mice. Motivated by these exciting data, the team now seeks to deliver this treatment to the patient within a “Single Patient Study” clinical trial design. Support from BioCanRx will enable the trial by funding clinical- grade manufacturing and preclinical validation of the clinical product, with the final outcome being the treatment of this patient.
If successful, the study will have a significant impact on the life of our patient and her family. It will also demonstrate the feasibility of manufacturing and delivering a novel solid tumour- targeting CAR T therapy to a cancer patient, its safety in a human being, and its antitumor activity against ASPS. This could prompt a larger, phase I clinical trial in ASPS patients across Canada.
More broadly, the study could demonstrate feasibility for the nonprofit development of personalized CAR T cell therapy of a rare orphan cancer.