Project summary: Clinical, Social and Economic Impact Program
Assessing the Real-World Clinical and Economic Outcomes of Emerging Innovative Technologies in Oncology: The Cases of Biosimilars and CAR T-cells
April 1st, 2019 to March 31st, 2022
HIGHLIGHTS

- Will use Real-World Evidence to advance innovations in health care and health technology assessments.
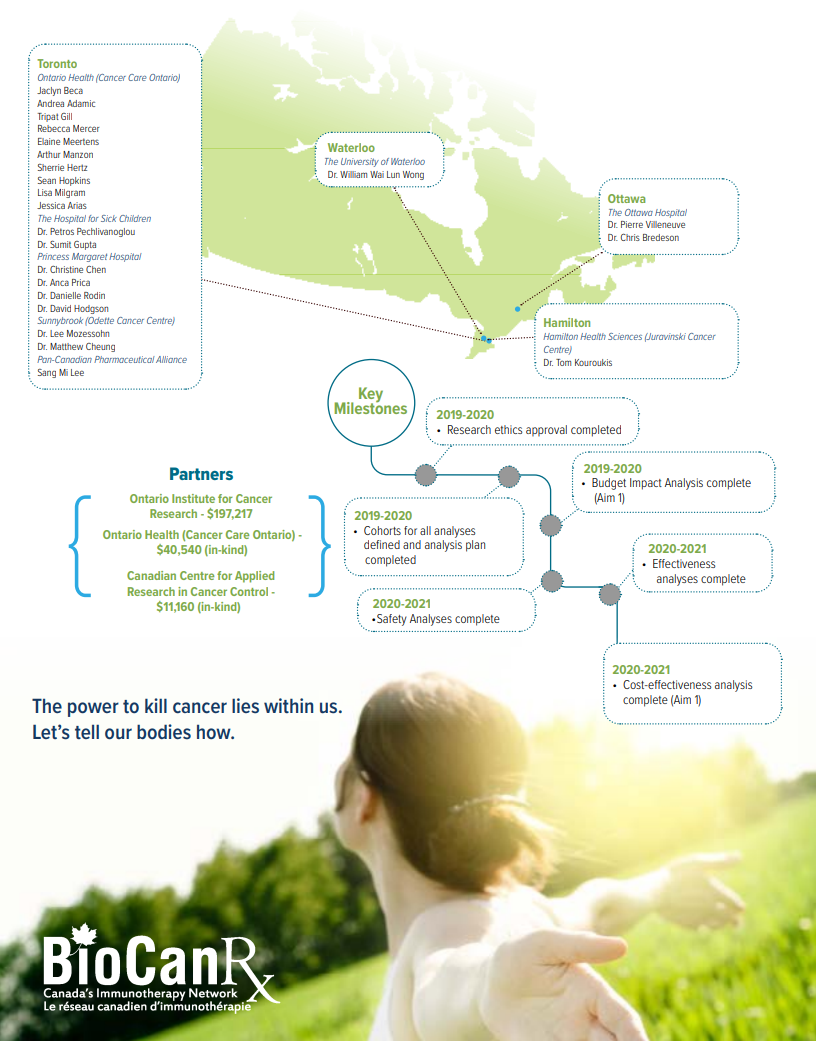
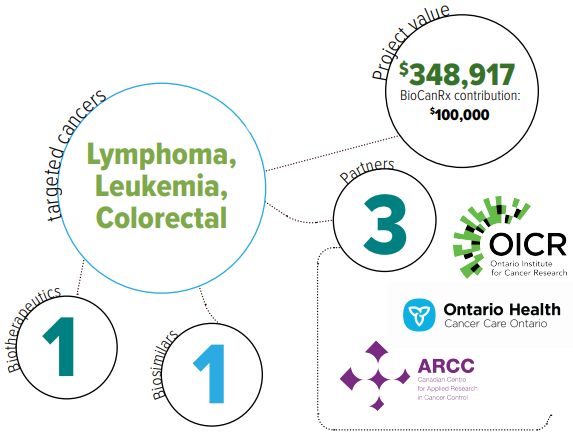
- Aim 1: To examine the real-world uptake, safety, effectiveness and economic impact of the implementation of biosimilar bevacizumab in advanced colorectal cancer.
- Aim 2: To evaluate the real-world health outcomes and economic impact of CAR T-cell therapy
About the Project
New cancer therapies on the horizon provide great promise to cancer patients, however, these new technologies are often very expensive and resource intensive in a health system already strained. Two examples of these new therapies include biosimilars and Chimeric Antigen Receptor T-cell (CAR T-cell) therapy.
Biosimilars are biologic medical products that are equivalent, though not identical, to original biologics that are manufactured by different companies when original biologic patents expire. Biologics are often very expensive, with biosimilars promising substantial cost-savings once available.
While extremely promising for both pediatric leukemia and adult lymphoma populations in terms of efficacy, CAR T-cell therapy is very costly, and has significant toxicity and related resource needs associated with it.
Understanding the real-world uptake, budget impact, effectiveness, safety, and cost-effectiveness of these new technologies is critical for system planning, resource allocation, and especially, ensuring the best patient care possible. We will conduct real-world evaluations of biosimilar bevacizumab and CAR T-cell therapy, evaluating uptake, health outcomes, and economic impacts in the Ontario landscape. The evidence generated by these evaluations can then be used to make informed decisions regarding the real-world impact of these new technologies, and to help develop proper policies and guidelines to ensure the best evidence based care for people with cancer.