BioCanRx Impact
Advancing Therapeutic Development
Cancer is a complex, highly heterogeneous disease, leading to inter-patient variability in treatment outcomes. BioCanRx’s research mandate is to fund promising research in cancer immunotherapy and to aim to extend successes in the clinic beyond initial responders. Since the inception of BioCanRx, we have advanced 34 novel therapeutics and approaches inclusive of three manufacturing process enhancements via our pipeline approach to funding. These novel therapeutics are targeting 40 cancer indications, inclusive of hard-to-treat cancer types and refractory and relapsed cancers. To date, 299 patients have been treated in 12 clinical trials examining the safety and efficacy of these of novel therapeutics and biotherapeutic approaches. Additionally, results of funded research projects will likely produce results that will benefit and extend to other cancer indications.
BioCanRx-funded projects aim at understanding patient and/or tumour type limitations and propose improved novel therapies, trials, or combination approaches to overcome the identified limitations. This will be accomplished by:
Identifying solid tumour targets for adoptive cell therapy
Turning “cold” tumours “hot”
Developing or refining manufacturing innovations
Developing or refining combination approaches
Identifying or validating mechanisms of resistance of tumours to immunotherapy in the clinical setting (cellular, immunological, genomic, biomarkers, pathway obstacles, microenvironment)
Biologically relevant cancer targeting (pathways, tumour microenvironment elements, immune cell subsets)
BioCanRx’s portfolio includes investments in three areas of cancer biotherapeutics research — oncolytic viruses & vaccines, cellular therapies, and antibodies and antibody-like molecules. We have a fourth field of research that assesses the societal value and economic viability of new therapies in development. All these research fields are supported by critical infrastructure required to move these technologies forward.
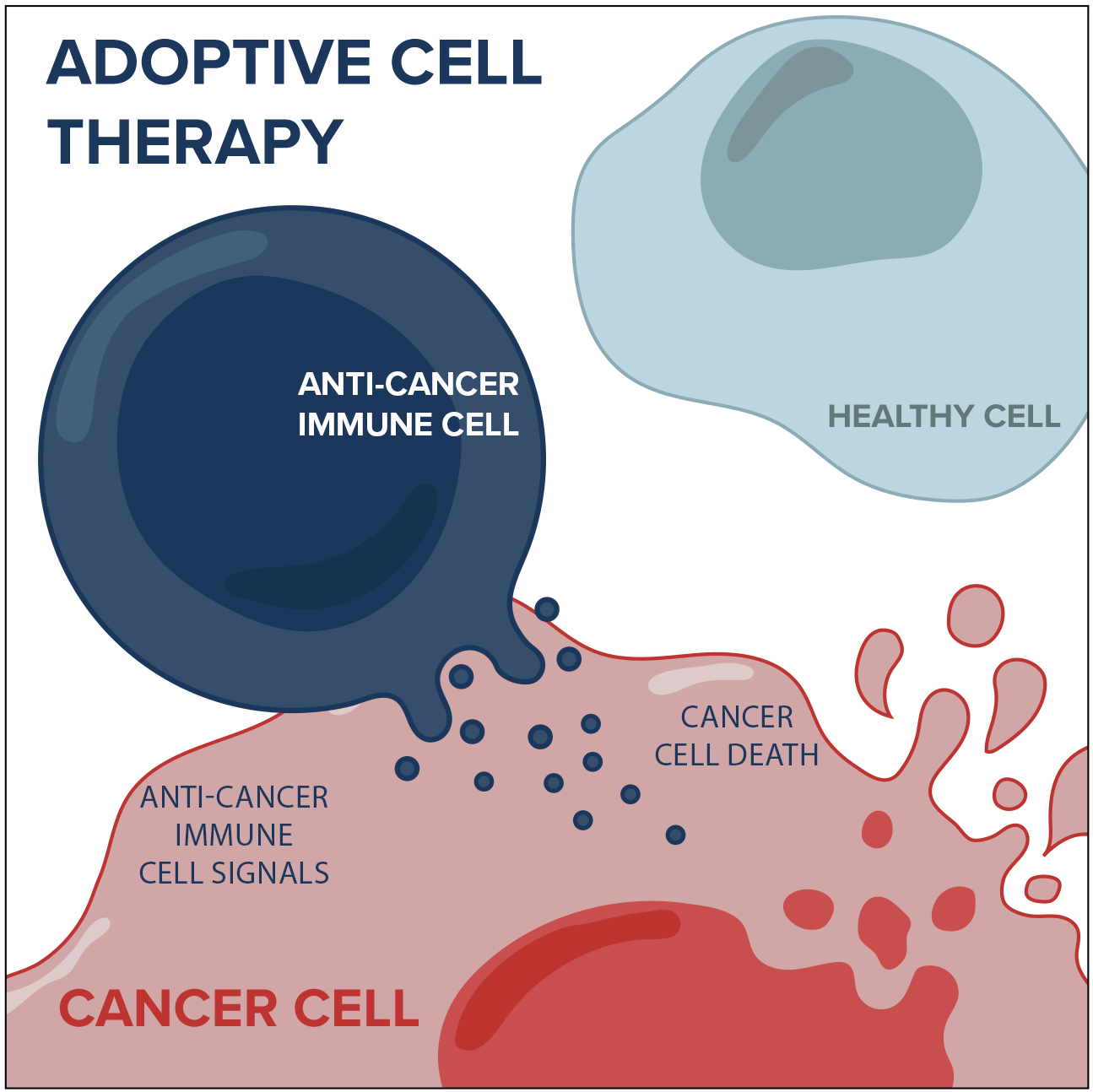
Adoptive cell therapy involves engineering or isolating cancer-fighting immune cells from a patient’s tumour, growing large numbers of these cells in the laboratory, and then infusing them back into patients. Immune cells are naturally present in most tumours, but usually lack the strength or numbers to eradicate the cancer on their own. The immune cells can also be genetically or biologically manipulated to become more therapeutically effective. This approach has led to some unprecedented clinical responses in patients with advanced cancers.
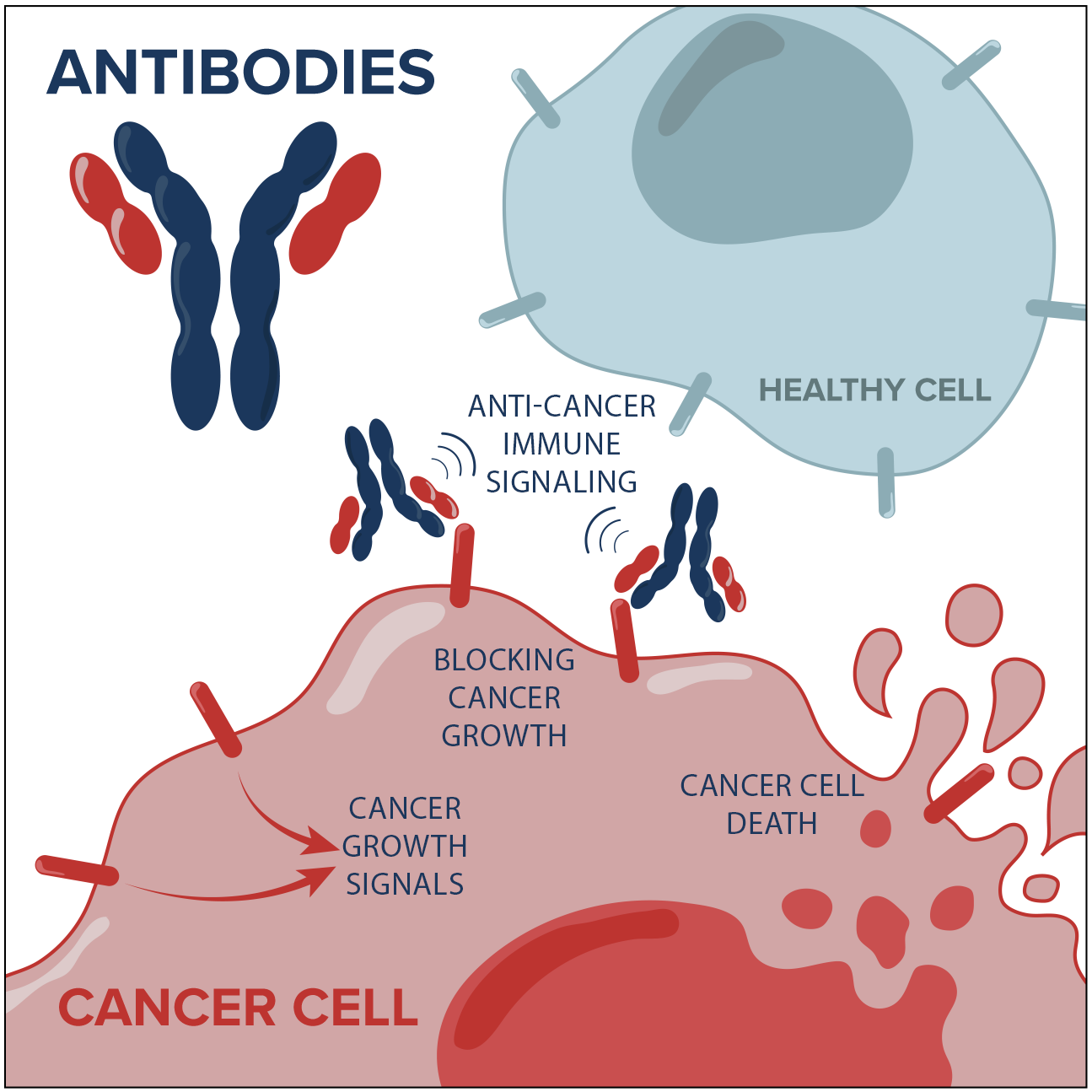
Natural antibodies are small proteins that the body produces to flag viruses, bacteria and cancer cells for destruction by the immune system. BioCanRx scientists are developing synthetic antibodies armed with potent toxins that can kill cancer cells directly, as well as antibodies directed against key immune regulatory checkpoints to drive the patient’s immune response towards heightened anti-cancer activity. These kinds of antibodies have already shown great promise in the clinic, and are without doubt the most successful anti-cancer biotherapeutics to date.
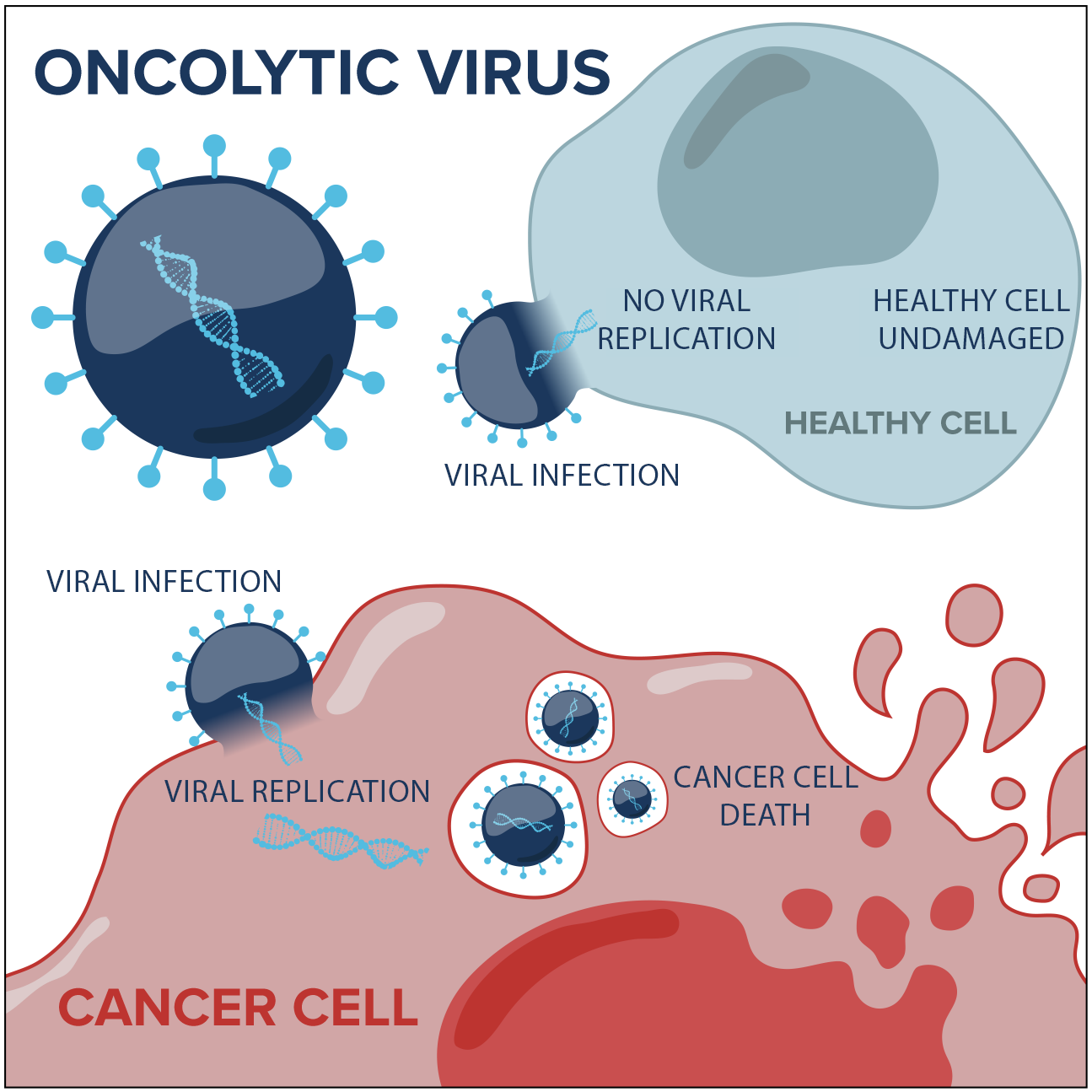
This highly innovative approach to cancer treatment uses cancer-killing viruses to target, infect and kill tumour cells. At the same time, these viruses train our immune system to identify cancer cells, which can provide lasting anti-tumour responses so the cancer doesn’t spread and recur.
Supporting Novel CAR T Therapies
BioCanRx is playing a lead key role in a Made-in-Canada CAR T program by investing in point-of-care cell therapy manufacturing facilities across Canada. In parallel to this investment are ones made in the research program. Starting in 2017, BioCanRx supported several projects related to the development of a CD19-targeting therapy that culminated in a Clinical Trial project, “CLIC-1901” for the treatment of patients with relapsed/refractory CD19 positive hematologic malignancies. The therapeutic product was fully made in Canada – with the clinical-grade plasmids developed in the GLP lab of Dr. Robert Holt at BC Cancer, the lentiviral vector manufactured at the Biotherapeutics Manufacturing Centre at the Ottawa Hospital Research Institute, and the final cell product manufactured at Dr. Brad Nelson’s facility at BC Cancer in Victoria. The CLIC-1901 process serves as a model for a fully Made-in-Canada approach that will be scaled out for other CAR T products. In its Cycle 2 of funding, BioCanRx has invested in the development of a novel CD22-targeting CAR that will be enabled using the CLIC-1901 model.

