Project summary: Enabling Study
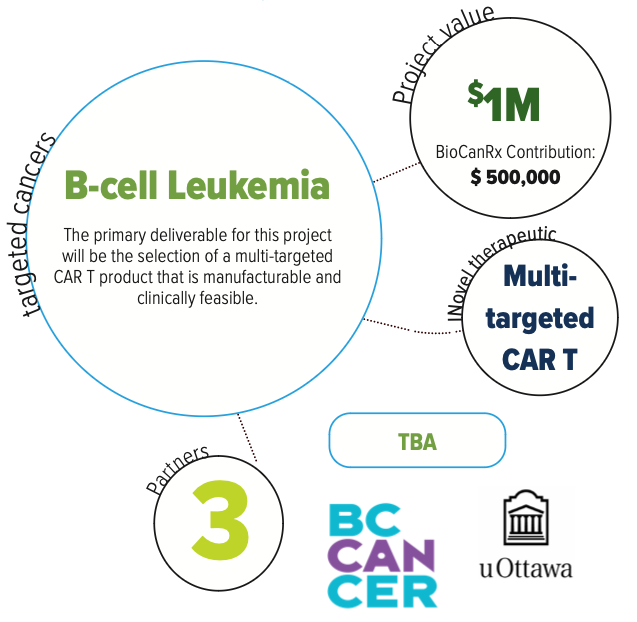
Clinical Trial Enabling Studies for Multi-targeted Chimeric Antigen Receptor Therapeutics for the Treatment of B-Cell Malignancies
October 1, 2020 to September 30, 2023
HIGHLIGHTS

- The project is expected to have a significant impact on the development of CAR T therapies in Canada by advancing highly- innovative multi-targeted and publicly owned therapeutic molecules to clinical trials.
- This project aligns with another Enabling Study by Dr. Kevin Hay and team, who are developing a CD-22 CAR T.
- The use of multi-targeted CAR T therapies is increasingly considered a necessary step to expand the indications for which CAR T is an effective option.
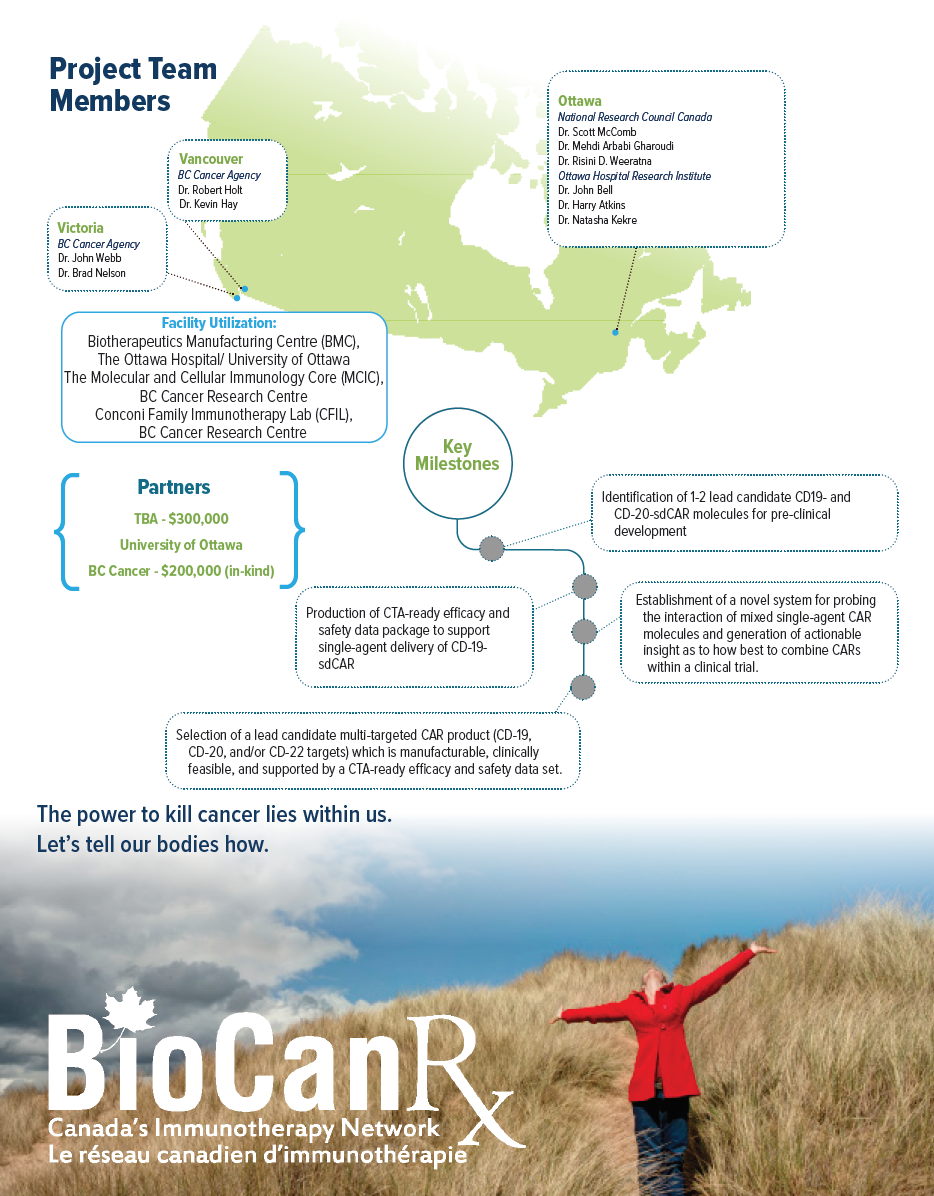
- The NRC and BioCanRx investigators have partnered to create a “target ID-to-CTA ready CAR T” development pipeline, with deliverables including a CTA-ready package to support single-agent delivery of CD-19- sdCAR and/or CD20-sdCAR, and selection of a lead candidate multi-targeted CAR product (CD-19, CD-20 and/or CD-22) single domain antibody targeted CAR T clinical trial.
About the Project
Chimeric antigen receptor therapies (CAR T) are revolutionizing the treatment of late stage B-cell leukemia, but there is still a long way to go; while CAR T cures some patients, relapse remains a critical issue. Instead of targeting a single antigen expressed on leukemia cells, as is the case for current-generation CD-19-targeted therapies, targeting multiple leukemia antigens is seen as an attractive strategy to offer better treatments to more patients. The researchers plan on exploiting the unique properties of antibodies derived from llamas and related camelid species (known as single-domain antibodies) to make an improved CAR T therapy that targets multiple antigens
expressed on B-cell leukemia.
Work has been performed at the National Research Council of Canada to generate new single domain antibodies that can bind to the CD-19, CD-20, and CD-22; receptors that are commonly expressed on many forms of B-cell leukemia. Preliminary testing has also been done to show that these new antibodies can make CAR T-cells that exhibit target-specific killing of leukemia cells in vitro and in vivo using murine models of human xenografts.
In this study, the researchers will explore several strategies of combining CD-19, CD-20, and CD-22 to make a therapy that can best eliminate leukemic cells from a mouse model of CAR T therapy. They will identify a lead candidate and develop a dataset demonstrating how this can be used safely and effectively to treat leukemia in Canadian patients.
Dependent on the outcome of the proposed work and availability of additional funding, the researchers intend to proceed to GMP manufacture and CTA document preparation shortly after the period of the grant.