COV-IMMUNO- A randomized phase III trial of vaccination with IMM-101 versus observation for the prevention of serious respiratory and COVID-19 related infections in cancer patients
Key Information
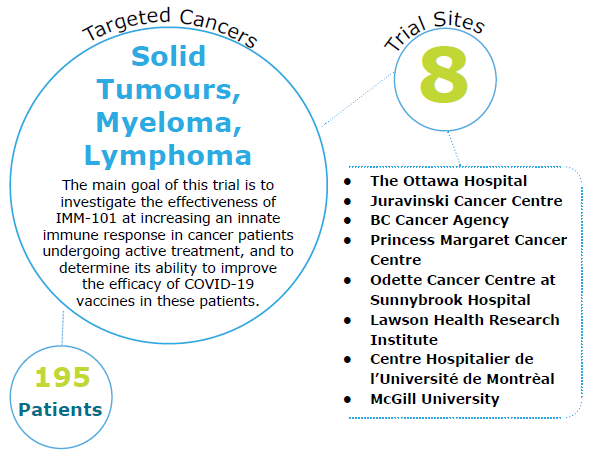
Who may qualify?
- Cancer patients with solid malignancies undergoing active cancer treatments including chemotherapy, targeted therapy, radiation or any other therapy that requires regular visits (i.e. > twice per month) to the cancer centre/hospital.
Recruitment status
- Active, not recruiting
Key words
- COVID-19, IMM-101, trained immunity, innate immune activation, vaccine
About the Trial
This trial has been developed to address a critical and urgent need to protect cancer patients during the COVID-19 pandemic. Cancer patients are particularly vulnerable to severe COVID-19 infections because they are immunocompromised and also can’t adhere to strict quarantine as they need to visit the hospital regularly for treatment.
Stimulation of the innate immune system (known as “trained immunity”) is a promising approach to optimizing the body’s response to many infections, including COVID-19. This principle has been shown in the past with recipients of the tuberculosis vaccine, known as BCG, as they demonstrated an increased resistance to other infections due to stimulation of their innate immunity. While BCG vaccination is being tested against COVID-19 in clinical trials around the globe, because it is composed of live bacteria, it can’t be used in patients with a weakened immune system, such as cancer patients.
In contrast, IMM-101, the investigational drug for this trial, is a whole cell immunomodulator that is safe to use in cancer patients because the bacteria have been killed. Given that it is NOT a live vaccine, it is being developed as an anti-cancer therapy, based on the above rationale of trained immunity, but against cancer cells. IMM-101 has been shown to induce an innate immune response in cancer patients of equal or greater magnitude to that reported with BCG treatment.
The purpose of this trial is to examine 1) IMM-101 impacts on the overall innate immune response/”immune training” of patients with cancer undergoing active treatment 2) the immune response to the COVID-19 vaccines in cancer patients on active therapy, 3) whether IMM-101 can improve the immune response of the COVID-19 vaccines or other vaccines, especially patients on immunosuppressive therapies.
For specific information to share with your doctor and care team click here.
Clinical Trial #: NCT04442048

