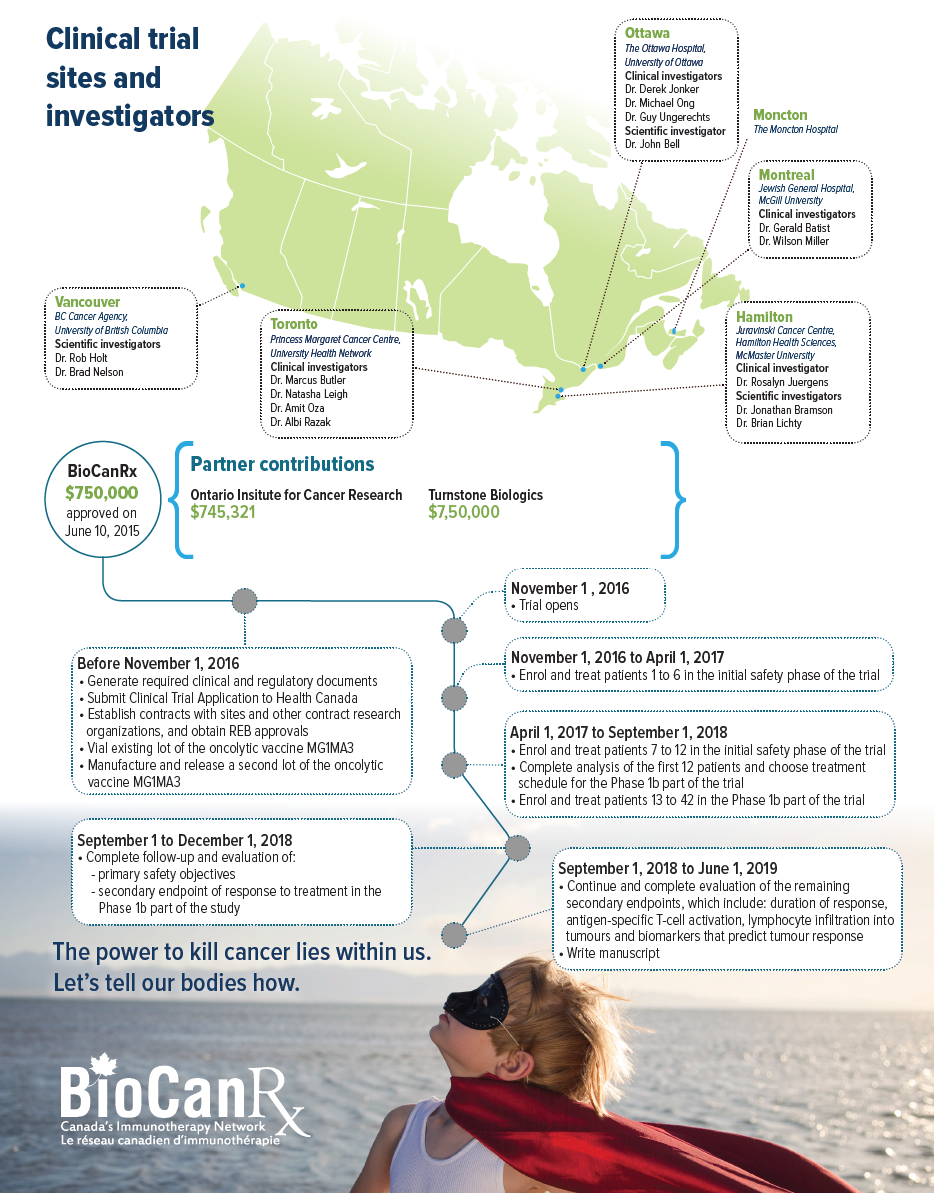
Project summary: Clinical Trials Program
Clinical trial to test the oncolytic vaccine approach in combination with checkpoint inhibitor antibodies
June 10, 2015 to June 30, 2019
HIGHLIGHTS

- World’s first clinical trial to combine an oncolytic vaccine approach with checkpoint inhibitor antibodies for cancer treatment
- The oncolytic vaccine strategy using adenovirus and Maraba virus was developed in Canada and its clinical testing remains exclusive to Canada
- Tremendous prospect for multi-sector partnerships at an early stage of testing
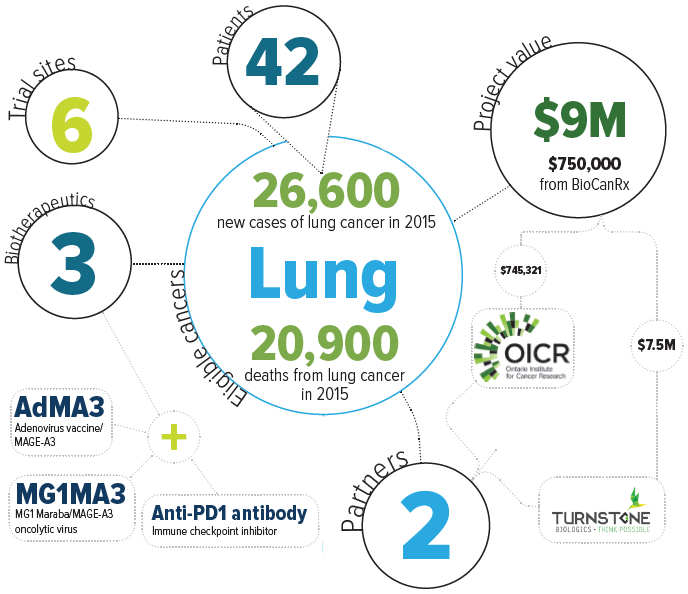
ABOUT THE PROJECT
This Phase Ib clinical trial will test a new biotherapeutic combination strategy in patients with advanced solid-tumour cancers that express the tumour antigen MAGE-A3 and have failed to respond to conventional therapies. The study will evaluate the safety, biology and anti-tumour activity of an approach that combines oncolytic virus vaccines with therapeutic antibodies. The oncolytic virus approach uses two viruses that, together, stimulate anti-tumour immune response and provide their own ability to kill cancer cells. Onto this, the trial will layer an antibody therapy in the form of an immune checkpoint inhibitor. These inhibitors target our immunological brakes, which normally function to hold the immune system at bay in order to avoid its over-activation against normal cells. These immunological brakes are often co-opted by cancer cells, allowing the cancer to escape detection by the immune system. By using immune checkpoint inhibitors to disrupt this deception, the immune system can properly detect the cancer and do its job to get rid of the disease.
Because only some patients in clinical trials respond to therapeutic antibodies on their own, it’s thought that immune checkpoint inhibitors are most effective in patients with an existing anti-cancer immune response. As a result, there is a search for agents that will sensitize cancers to immune checkpoint inhibitors. This trial will explore whether the proposed oncolytic virus vaccine approach will sensitize the cancer in this way, while also delivering its own cancer-killing properties.