Project dashboard: Clinical Trials Program
CLINICAL TRIAL TO TEST THE ONCOLYTIC VACCINE APPROACH IN COMBINATION WITH CHECKPOINT INHIBITOR ANTIBODIES
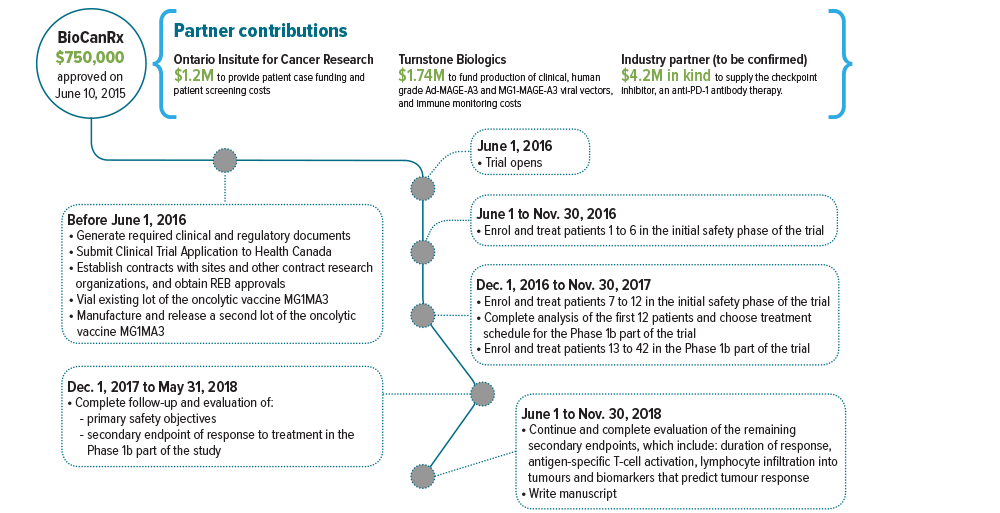
June 1, 2016 to Nov. 30, 2018
HIGHLIGHTS

- World’s first clinical trial to combine an oncolytic vaccine approach with checkpoint inhibitor antibodies for cancer treatment
- The oncolytic vaccine strategy using adenovirus and Maraba virus was developed in Canada and its clinical testing remains exclusive to Canada
- Tremendous prospect for multi-sector partnerships at an early stage of testing
ABOUT THE PROJECT
This Phase Ib clinical trial will test a new biotherapeutic combination strategy in patients with advanced solid-tumour cancers that express the tumour antigen MAGE-A3 and have failed to respond to conventional therapies. The study will evaluate the safety, biology and anti-tumour activity of an approach that combines oncolytic virus vaccines with therapeutic antibodies. The oncolytic virus approach uses two viruses that, together, stimulate anti-tumour immune response and provide their own ability to kill cancer cells. Onto this, the trial will layer an antibody therapy in the form of an immune checkpoint inhibitor. These inhibitors target our immunological brakes, which normally function to hold the immune system at bay in order to avoid its over-activation against normal cells. These immunological brakes are often co-opted by cancer cells, allowing the cancer to escape detection by the immune system. By using immune checkpoint inhibitors to disrupt this deception, the immune system can properly detect the cancer and do its job to get rid of the disease.
Because only some patients in clinical trials respond to therapeutic antibodies on their own, it’s thought that immune checkpoint inhibitors are most effective in patients with an existing anti-cancer immune response. As a result, there is a search for agents that will sensitize cancers to immune checkpoint inhibitors. This trial will explore whether the proposed oncolytic virus vaccine approach will sensitize the cancer in this way, while also delivering its own cancer-killing properties.
SCIENTIFIC INVESTIGATORS
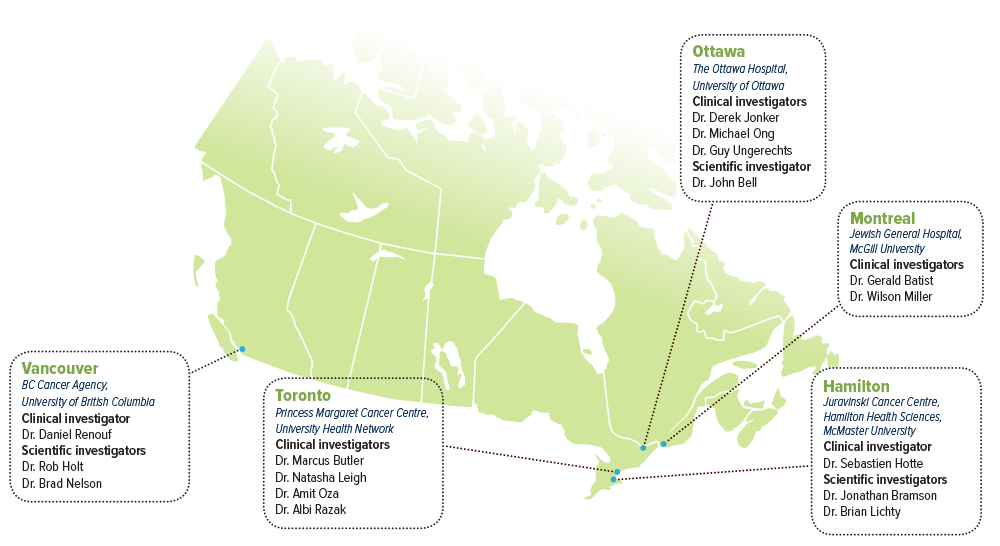
- Dr. John Bell, The Ottawa Hospital, University of Ottawa
- Dr. Jonathan Bramson, McMaster University, Juravinski Cancer Centre, Hamilton Health Sciences
- Dr. Rob Holt, BC Cancer Agency, University of British Columbia
- Dr. Brian Lichty, McMaster University, Juravinski Cancer Centre, Hamilton Health Sciences
- Dr. Brad Nelson, BC Cancer Agency, University of British Columbia
CLINICAL ADVISORS
- Dr. Gerald Batist, Jewish General Hospital, McGill University
- Dr. Marcus Butler, Princess Margaret Cancer Centre, University Health Network
- Dr. Sebastien Hotte, Juravinski Cancer Centre, Hamilton Health Sciences, McMaster University
- Dr. Derek Jonker, The Ottawa Hospital, University of Ottawa
- Dr. Natasha Leigh, Princess Margaret Cancer Centre, University Health Network
- Dr. Wilson Miller, Jewish General Hospital, McGill University
- Dr. Michael Ong, The Ottawa Hospital, University of Ottawa
- Dr. Amit Oza, Princess Margaret Cancer Centre, University Health Network
- Dr. Albi Razak, Princess Margaret Cancer Centre, University Health Network
- Dr. Daniel Renouf, BC Cancer Agency, University of British Columbia
- Dr. Guy Ungerechts, The Ottawa Hospital, University of Ottawa
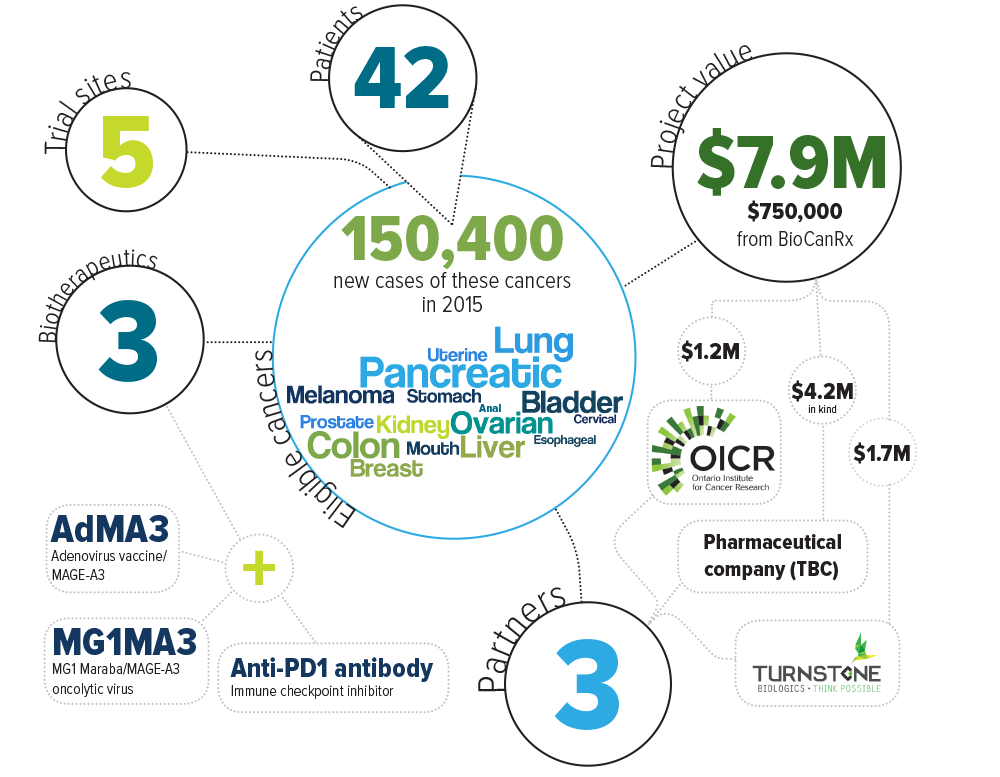
Keywords: oncolytic virus, oncolytic vaccine, antibody, immune checkpoint inhibitor, adenovirus vaccine, Maraba oncolytic virus, pembrolizumab, AdMA3, MG1MA3
Eligible cancers: anal, bladder, breast, cervical, colon, esophageal, kidney, liver, lung, melanoma, mouth, ovarian, pancreatic, prostate, stomach, uterine
Partners: Ontario Institute for Cancer Research, Turnstone Biologics, Industry partner to be confirmed