Project summary: Clinical, Social and Economic Impact
Making Patient Partnerships A Reality in Very Early Phase Clinical Trials (MARVEL): Development of a Patient Engagement Platform
October 1, 2020 – April 1, 2023
HIGHLIGHTS

- Patient engagement in early phase clinical trials holds great promise for impact. However, very few clinical trials report on the involvement of patients as partners.
- This project is unique in that it will encompass clinical programs at various development stages (i.e. late-stage development programs, and trials that are enrolling or about to enroll).
- Publications describing the resulting frameworks and processes, evaluations, and comparisons across trials, will help to advance the field of patient engagement; in turn this may inspire other researchers within the cancer biotherapeutics scientific community to engage patients.
About the Project
When developing clinical trial ideas and protocols, investigators need to bring to the table the most important group of stakeholders: the patient, their family and caregivers. Researchers have highlighted numerous potential benefits to patient engagement, including selection of more relevant outcomes, help in refining eligibility criteria, improving the clarity of informed consent documents and providing patient perspectives on the feasibility of interventions. Despite this, reports of patient engagement in clinical trials have been rare.
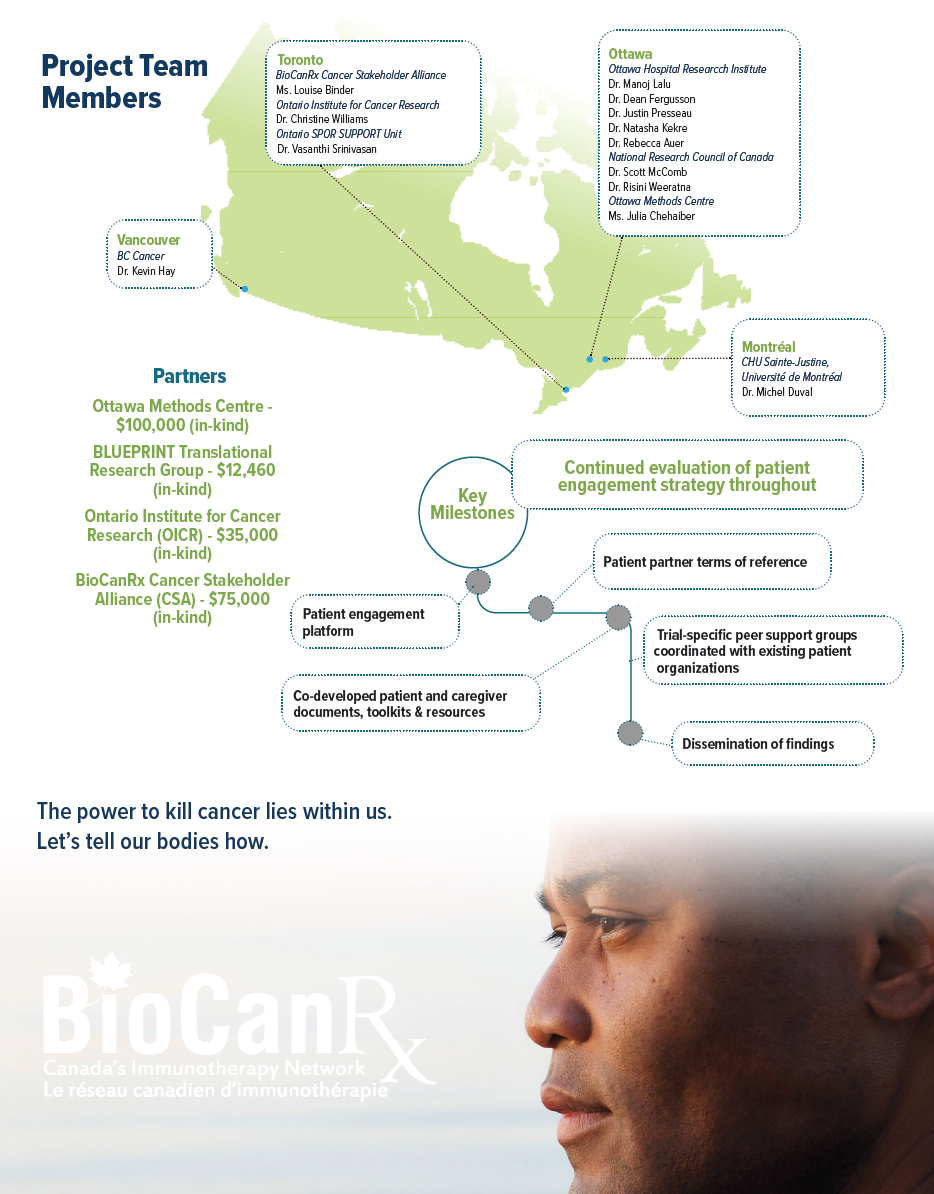
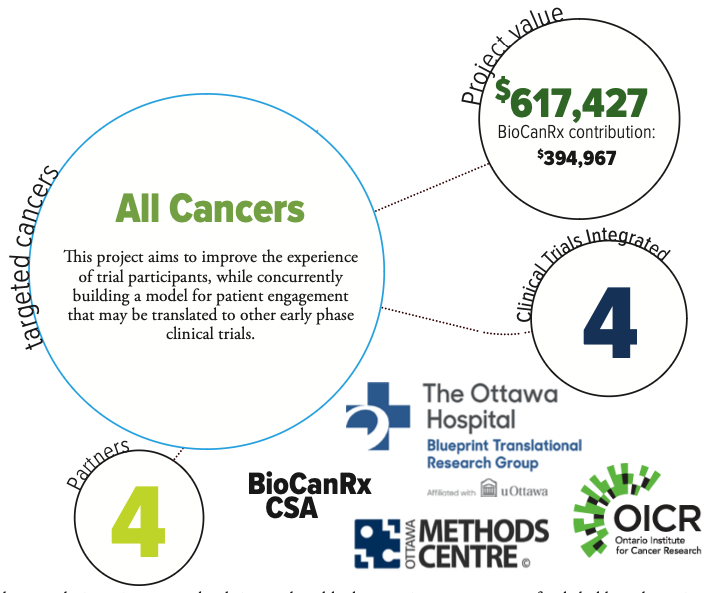
The project aims to address this gap through development of a patient engagement platform for early phase clinical trials. The researchers will directly engage patients as partners in five BioCanRx-funded cancer immunotherapy programs. Patients will be from across Canada and will have access to bilingual staff to ensure equitable opportunity for French and English-speaking populations.
Working alongside the research team, patient partners will help to identify trial participant preferences and needs, and will provide input on study outcome measures and the development of the trial protocol.
A longitudinal evaluation of patient engagement strategies is planned to assess impact, areas for improvement and patient and researcher perspectives on the approaches taken. This work will allow for a comprehensive synthesis; the researchers will compare and contrast patient engagement strategies implemented across five trials at different development stages (e.g. late-stage product development, trial protocol development, ongoing recruitment).
Building upon the Go-CART project funded in Cycle 1, this research will help to provide conceptual frameworks, approaches, toolkits, and best practices for engaging patients in early phase clinical trials. By involving patient partners, the selected trials will be better-aligned with patient priorities and needs, and equip participants with appropriate resources and address modifiable barriers.