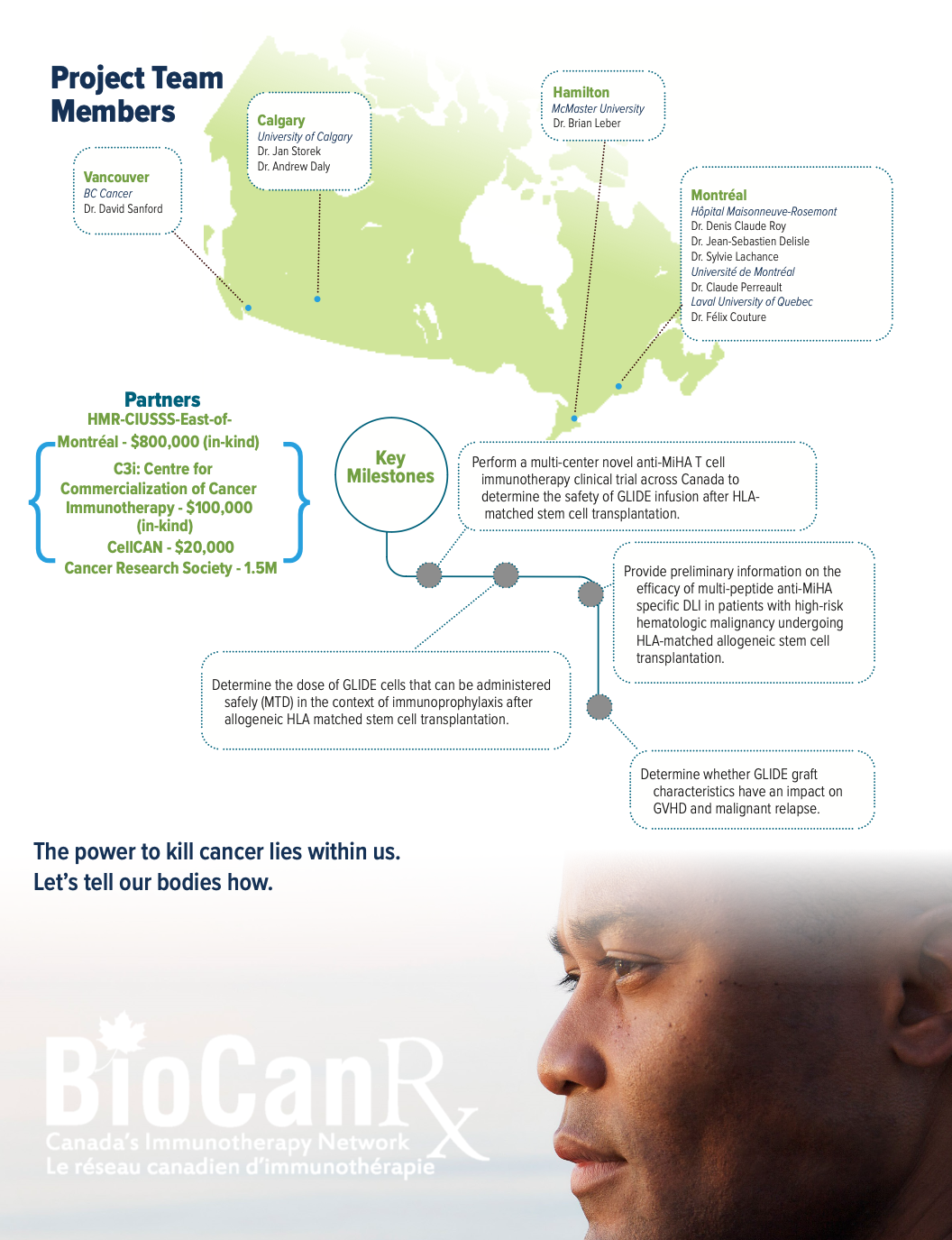
Project summary: Clinical Trial
An Interventional, Open-label, Non-randomized Dose-escalation Phase I Multicenter Clinical Trial of Multi-peptide Anti-minor Histocompatibility Antigen Immunotherapy for the Prevention of Relapse in Patients with High-risk Malignancy Eligible for Matched Related ASCT
July 1, 2020 – March 31, 2024
HIGHLIGHTS

- A “first-in-human” clinical trial in relapsing patients has already demonstrated signs of activity without severe toxicity. This trial will facilitate implementation and broaden target patient population.
- New and well-defined MiHAs that are i) expressed mainly/ only on hematopoietic cells, and ii) overexpressed on neoplastic cells, are used to generate an anti-MiHa donor lymphocyte infusion (DLI) with a manufacturing approach that avoids T cell exhaustion, to be administrated day+60 post ASCT.
- Companion tests with novel IP have also been developed and implemented under the Centre for Commercialization of Cancer Immunotherapy (C3i).

About the Project
Long-term remissions of hematologic malignancies after allogeneic stem cell transplantation (ASCT) rely largely on the graft-versus-leukemia effect (GVL). In a fully HLA-matched setting, the GVL effect is mediated by T cell immune responses against host minor histocompatibility antigens (MiHAs) found on malignant cells surface. 16-51% of AML patients experience recurrence of their disease within 2 years following AHCT. In patients with high-risk hematologic malignancy the GVL effect appears to be more limited and disease progression is known to be higher than 50%, questioning the benefit of this immunotherapy. Patients with relapsed acute leukemia or myelodysplastic syndrome following AHCT can be treated with their original donor lymphocyte infusions (DLI), but only 10-30% of them achieve remission.
The ability to target MiHAs preferentially expressed on leukemia cells is a promising strategy to prevent post ASCT relapse. In this study, the researchers will use a novel immunotherapeutic strategy by selecting 98 MiHAs preferentially expressed on hematologic cancers cells over other tissues, to generate anti-MiHA T cells line (GLIDE) while minimizing the risk of GVHD.
Ten patients have already who relapsed post ASCT have already been treated with 1-2 infusions of single (n=9) or multi (n=1) anti-MiHA peptide stimulated T cells in a multicenter phase I study. This treatment has demonstrated signs of activity without toxicity. Following this proof of concept, the researchers plan to generate a DLI targeting multiple MiHA peptides to be administrated simultaneously day+60 post ASCT in patients with high-risk hematologic malignancies to increase the GVL effect and improve patient long-term outcome.
This application is addressing priority areas of BioCanRx: it is investigating a novel immunotherapeutic strategy for high risk blood cancers, using biologically relevant anti-MiHA cancer targeting DLI, developed with sophisticated genomic and immunologic approaches, incorporating multiplex ex vivo expansion strategy, and assessing its activity (HTA) in a clinical trial setting. Most importantly, it is gathering efforts from major centers across Canada.