BioCanRx Impact
Improving Accessibility
Emerging biotherapeutic products and platforms are transforming the field of cancer immunotherapy. These innovations are providing new hope for cancer care. But they can face many social, legal, ethical, economic or health-systems barriers as they progress through the translational pipeline from preclinical research to clinical trials and beyond.
To help develop potential solutions to these challenges, BioCanRx created the Clinical, Social and Economic Impact (CSEI) program. It ensures our pipeline of funded research and clinical projects is collecting “the right data, in the right way, at the right time.” These projects address what is needed to successfully develop new therapies. The CSEI program enables the successful translation and adoption of cancer immunotherapy innovations by:
- Supporting translational science to increase efficiency of translational research by understanding the scientific and operational principles underlying each step of the translational process;
- Evaluating and positioning new immunotherapies under development for future uptake in the Canadian health care system; and
- Examining Canadian regulatory policy challenges that impact the path of curative therapies from bench to bedside
Supporting Translational Science
The entry of novel therapies in the bench-to-bedside pipeline can be challenging. This usually has to do with research project design and methodology flaws. Robust and unbiased experimental design and methodology for analysis and interpretation of clinical translation studies are critical in ensuring rigor and reproducibility before taking the next translational step, and ultimately to ensuring that promising therapies make their way to Canadian patients. Through its CSEI program, BioCanRx supports best practices in this area.
Assessments of preclinical research have consistently found that key elements of research design (e.g., blinding and randomization) are missing from published work, which can lead to irreproducibility of findings, and ultimately to barriers in the development of new immunotherapies. To help overcome this issue, BioCanRx supported Drs. Dean Fergusson and Manoj Lalu (OHRI) via CSEI project funding to develop a workshop on Preclinical Experimental Design and Reporting targeted toward funded investigators undertaking preclinical research, which will continue to be offered.
Evaluating and Positioning Novel Biotherapeutics for Adoption into the Canadian Healthcare System
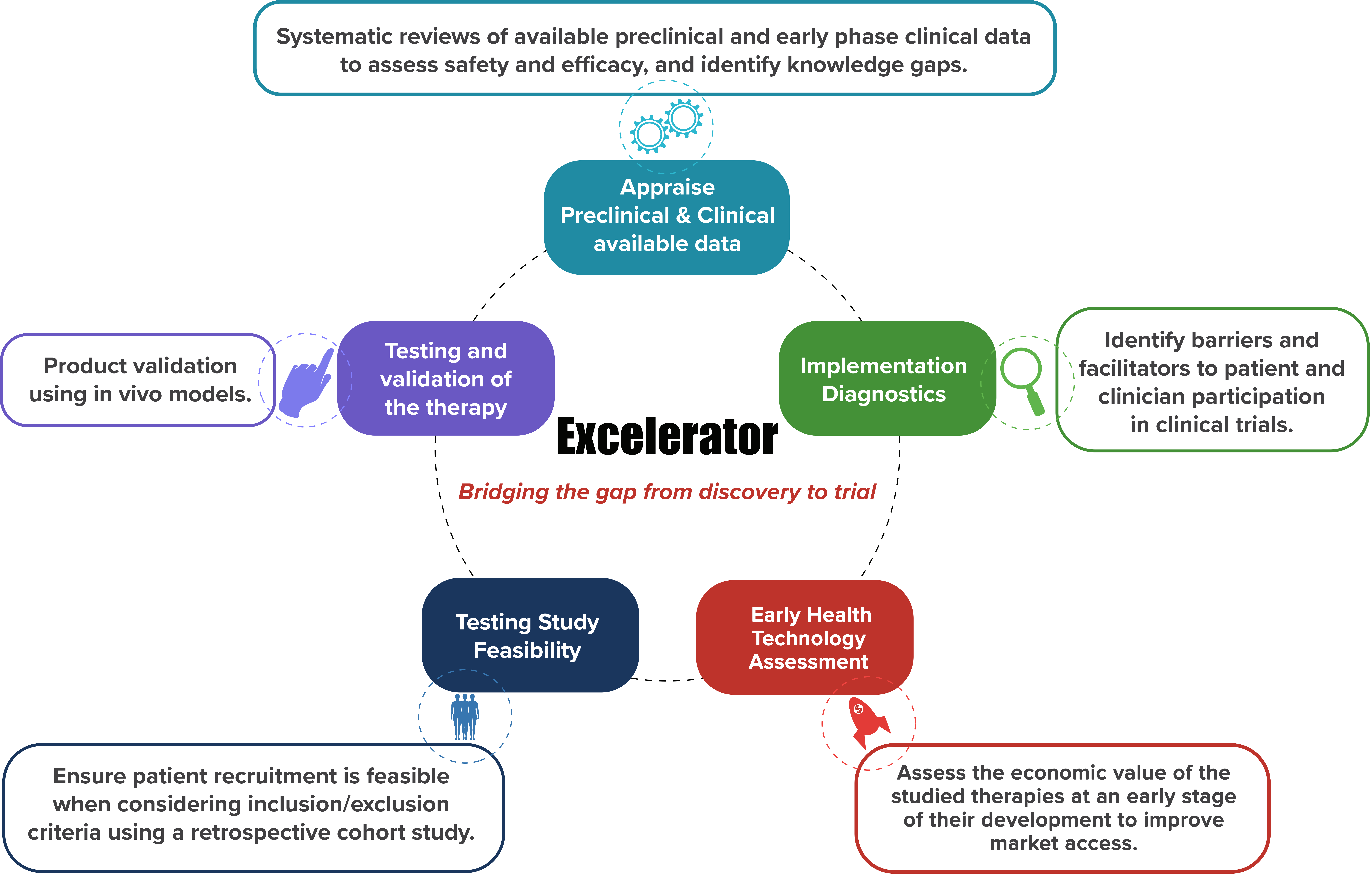
One CSEI project example is Getting Better Outcomes with Chimeric Antigen Receptor T cell therapy (GO–CART). It’s an integrated knowledge translation project demonstrating a critical mechanism to develop clinical trial protocols around novel technologies with multi-stakeholders (patients and oncologists) and to build upon a comprehensive evidence base. GO–CART has developed protocols for individual study components and a trial protocol; these were built with patient partners to ensure relevance to the target patient population. This clinical trial protocol design approach approach incorporating early health technology assessment (eHTA) early HTA is part of the BioCanRx CAR T-cell program platform.
Getting a Head-start with Early Health Technology Assessment (HTA)
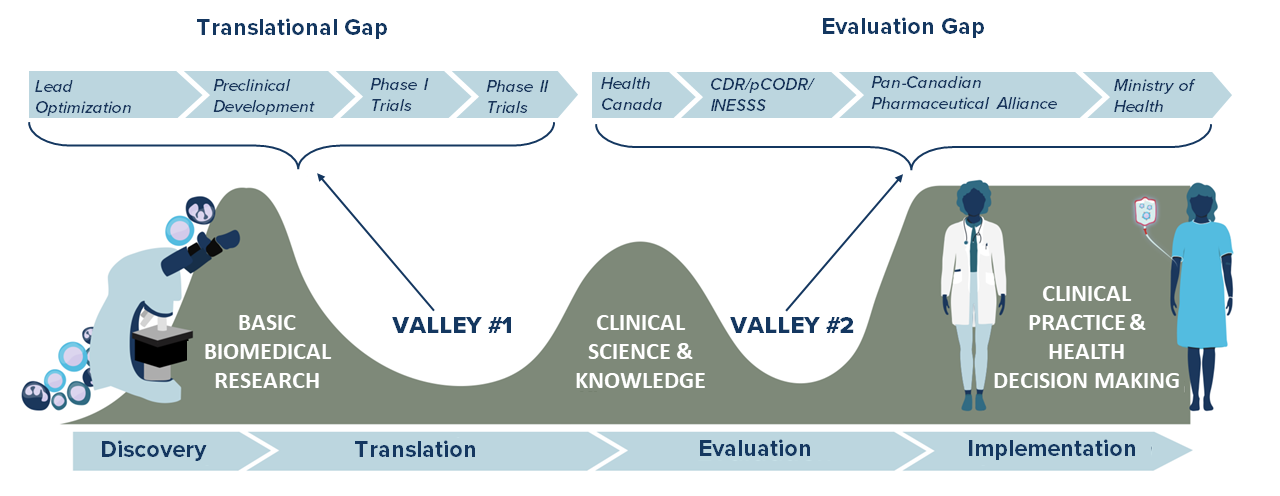
The pathway for bringing a new therapy to the market is dependent on two sequential processes: achieving market authorization from the regulatory agency (Health Canada) and reimbursement from a payer (a provincial health ministry). Carrying out these two related but different evaluations in sequence takes time. To provide more timely access to drugs, Health Canada developed an initiative that would allow alignment of these processes alongside Health Technology Assessment organizations such as the Canadian Agency for Drugs and Technologies in Health (CADTH) and l’Institut national d’excellence en santee et en services sociaux (INESS – Quebec). These processes take place at the later stages of a products development cycle (Phase III).
Early HTA is identified as the early assessment of safety, effectiveness, and cost-effectiveness profiles of new medical technologies based on evidence mainly derived from early-phase clinical research outcomes. Incorporating early HTA at the beginning stages of clinical research is an important step in informing various stakeholders on the potential value of a new therapy. It examines cost-effectiveness and feasibility of an innovation from the start, as demonstrated by the GO-CART project for CAR T-cell therapy.
The persistent need to assess the ‘true’ value of cancer treatments is increasingly prevalent in today’s climate of ballooning cancer care costs. The advent of highly personalized, exceedingly complex biologic therapies requires exhaustive economic evaluation to support healthcare decision makers and funding agencies in allocating limited resources. BioCanRx supports research that looks at collecting data at the early stages of clinical evaluation to inform stakeholders, including policy makers, about the potential value of new medical products in development.
Early HTA tools are applied to phase I Clinical Trial applications or funded projects in the BioCanRx therapeutics portfolio. Another example is Developing System-Level Policy Model for Regenerative Medicine and Cell Therapy in Oncology (Drs. Wong, University of Waterloo, and Chan, Sunnybrook Health Sciences Centre). It was a critical early HTA project to further inform the path to implementation for CAR T-cell therapy in Canada. It engaged regulators and payors to inform important policy questions related to the timeliness and use of CAR T cell therapies in Ontario patients. The outcomes of this work have been shared with the ultimate knowledge end-users: CADTH, Canada’s HTA body and Ontario Health.
Collecting Real-World Evidence to Inform Decision Making
Another CSEI project example is Assessing the Real-World Clinical and Economic Outcomes of Emerging Innovative Technologies in Oncology: The Cases of Biosimilars and CAR T-cells. Dr. Kelvin Chan looked at two kinds of cancer therapies – the use of a biosimilar, bevacizumab, to treat advanced colorectal cancer, and CAR-T cell therapy to treat leukemia in children and lymphoma in adults – to see how they behave in the real world. So how to get this real-world evidence? In Canada it’s easier than many other places, largely because our publicly funded health-care system means large patient and administrative data sets are available.
On a patient level, Dr. Chan followed patients from their initial enrollment to ascertain things like survival rates and side effects. He looked for outcomes like return to hospital after treatment and patients going to emergency. He also looked at patients’ baseline characteristics to learn more about who received these treatments and whether factors like co-morbidities influence outcomes. On a system level, Dr. Chan is looking at resource utilization – at costs, and at what factors drive up costs, or help bring them down. Successful treatment, for instance, can bring down overall costs because further treatments are not needed. Unsuccessful treatment, or treatment with significant side effects can drive up costs.
Working with Policy Makers to Streamline Regulations
An efficient regulatory system is critical in advancing translational research and building a vibrant life sciences ecosystem. At times, subtle regulatory change leads to unexpected results, as was the case with the inclusion of “virus-like particles or sub-viral particles” in the definition of New Substances Notification Regulations for Organisms (NSNR(O)), which impacted clinical cell and gene therapy and commercialization.
The requirement of an independent 120-day Environment and Climate Change Canada (ECCC) review preceding a Health Canada review on quality and environmental concerns placed an additional burden on sponsors submitting clinical trial applications (CTA) and/or New Drug Submissions (NDS). The first recommendation from this workshop and policy review was adopted by the Government of Canada, leading to the recommendation in Budget 2021 to integrate the environmental risk assessment with Health Canada submission stages, creating a single window for submitting data. This “evidence to policy to practice” BioCanRx-funded work led by Dr. Tania Bubela (SFU) serves as great example of streamlining regulations to provide greater efficiencies!

