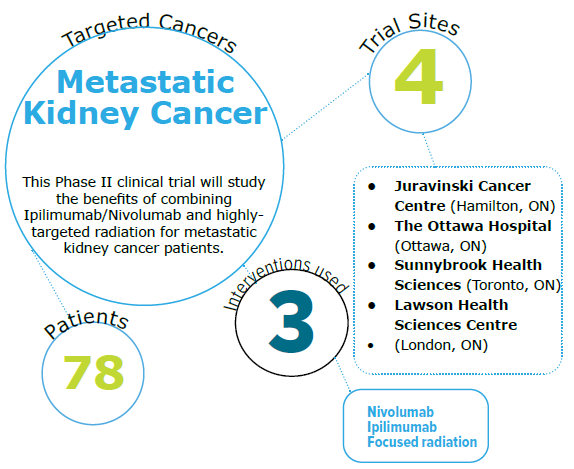
Phase II CYTOSHRINK Trial: Cytoreductive Stereotactic Hypofractionated Radiotherapy with Combination Ipilimumab and Nivolumab for Metastatic Kidney Cancer
Key Information
Who may qualify?
- Newly diagnosed and histologically confirmed intermediate/poor risk metastatic renal cell carcinoma (mRCC) patients
- Biopsy proven renal cell carcinoma of any histology; Imaging proven metastatic disease based on CT or MRI within 10 weeks of screening; Primary kidney lesion amenable to SBRT; Eligible for standard of care delivery of ipilimumab and nivolumab (I/N)
Recruitment status
- Actively recruiting
Key words
- Ipilimumab/Nivolumab, kidney cancer, radiation, Immune Checkpoint Inhibitor (ICI)
About the Trial
Treatment which turns on the body’s own immune system to attack cancer cells is called immunotherapy. One type of immunotherapy are checkpoint blockers, which work by blocking the “checkpoints” in our body. These “checkpoints” ensure our immune system is not overactive and to limit it from attacking our “self”. In the case of cancer, which can evade our immune system, blocking these checkpoints allow T cells to better kill cancer cells.
Immune checkpoint blockers, such as Ipilimumab and Nivolumab, have been shown to improve the lifespan of patients with metastatic kidney cancer by unleashing our immune system. However, not every patient benefits from immunotherapy. Understanding the factors that indicate which patients are likely to benefit from treatment, or ways to help immunotherapy work better for more patients, are important, unmet needs.
Removal of the primary kidney was shown over 20 years ago to be modestly beneficial for metastatic kidney cancer patients; however, recent studies cast doubt on this benefit in the current immunotherapy era. Alternatively, highly focused radiation is a convenient, safe method to kill cancer cells that may also enhance our immune response.
The researchers hypothesize that combining immunotherapy and highly focused radiation will improve the treatment of kidney cancer. To address this hypothesis, the researchers are conducting a clinical trial (CYTOSHRINK) to study the benefits of combining Ipilimumab/Nivolumab and radiation for metastatic kidney cancer patients. They will also gather information on how patients respond to immunotherapy plus radiation by studying changes in their blood and gut bacteria during treatment. Ultimately, improved combination therapies with better cancer control will significantly impact the quantity and quality of life for Canadian patients.
Clinical Trial #: NCT04090710

