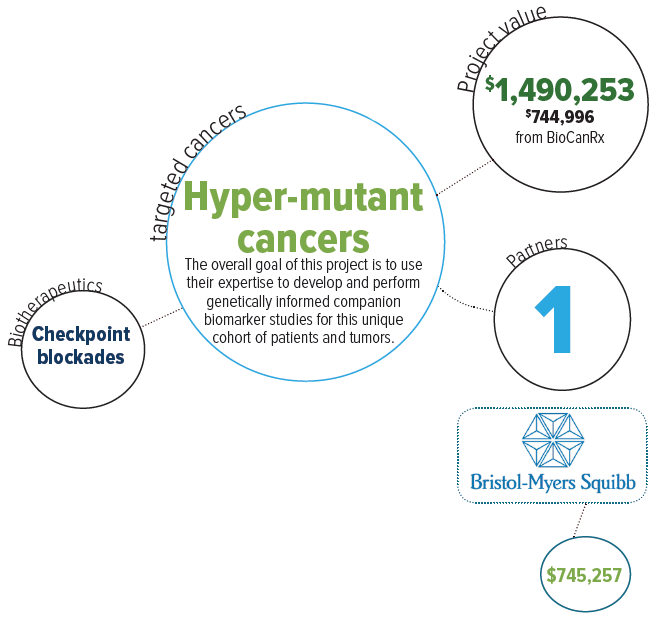
Project summary: Enabling Studies Program
Development of predictive companion biomarkers and therapeutic monitoring for hypermutant cancers to immune checkpoint inhibition
Oct 14, 2016 to Mar 31, 2021
HIGHLIGHTS

- Develops robust objective tools to predict which patients will be cured by immunotherapy and monitors tumour response using a non-invasive blood test
- Establishes a global leadership in companion diagnostic development for immunotherapy in hypermutant cancers
- Network collaboration will allow moving novel biomarkers to the clinical arena positioning Canadian clinician-scientists as leaders in the field
About this project
Immunotherapy is the most promising cancer therapy of the last decade. Although some previously incurable tumours can now be treated successfully, it is impossible to predict which patient will be cured and how to monitor tumour response to therapy. Immune checkpoint inhibition is emerging as the most promising therapy for some of the deadliest human cancers offering long term cures for 20-40% patients with melanoma, lung cancers and others.
The major challenge facing the scientific community is to figure out which of these patients will respond to immunotherapy. Efforts currently fail to detect biomarkers predicting a response to these therapies due to the complexity of these adult cancers. However, higher mutations in the tumour are associated with better response to immunotherapy, yet that correlation remains questionable. Strong biomarkers that can predict patient’s outcome or therapeutic effect are needed. This project aims to conduct a complete companion biomarker discovery and validation on their upcoming international clinical trial using immune checkpoint inhibition on these cancers.
This international endeavor led by Canadian PIs from the University of Toronto and the Hospital for Sick Children, has been assembled to actualize two major needs in immune checkpoint blockade therapies: to identify biomarkers of therapeutic response, and to establish consecutive blood tests for non-invasive monitoring and prediction of response. Enabling the translation of their initial findings to the development of robust biomarkers for PD-1 blockade in these patients as well as the corresponding common adult cancers is their goal.
In a previous project, the team uncovered a unique group of patients with ultrahypermutant cancers. They discovered that these cancers respond dramatically to immune checkpoint inhibition and will be perfect to study tumour and host related mechanisms responsible for the development of effective biomarkers for immunotherapy. The discovery that bMMRD cancers have the highest mutational load and respond favourably to immune checkpoint inhibition, puts this project in a unique and advantageous position to create a platform which will lead to advanced therapeutic applications in other common hypermutant cancers.
Based on this exciting data, they are performing an international clinicaltrial, using the anti-PD-1 biologic called Nivolumab to treat these “hypermutant” cancers. This first-of-its-kind trial includes patients enrolled from all over the world and all genetic data is gathered and processed in Toronto. Data from this project will position the team as leaders in the field and will allow for the development of robust biomarkers for future immunotherapies in common adult cancers.
Finally, the ongoing blood monitoring assays, using the novel tools, will allow this project to develop non-invasive tools which will transform the therapeutic and clinical approaches to these cancers. This project will spearhead future international clinical trials and position Canadian clinician-scientists as leaders in the field.


