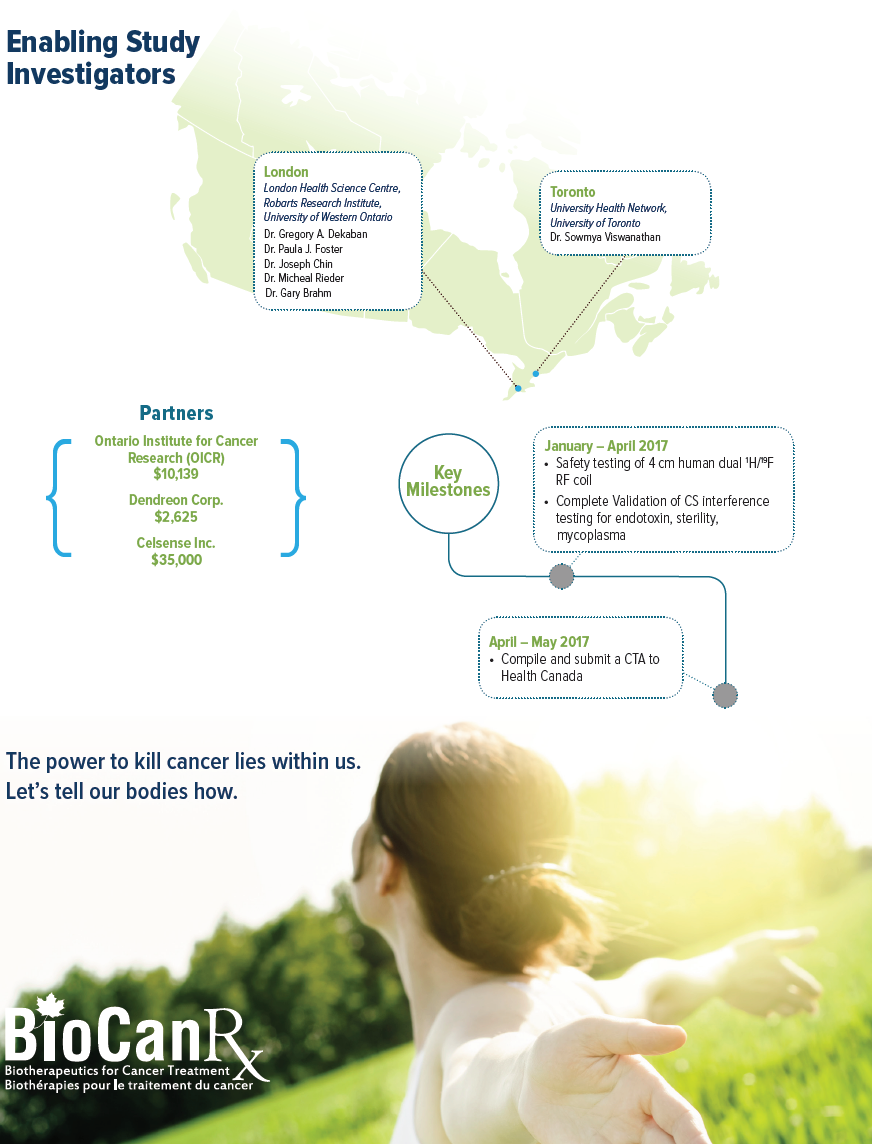
Project summary: Enabling Studies Program
In vivo human therapeutic cell tracking using a [19F]-perfluorocarbon cellular MRI contrast agent: Phase I feasibility and preliminary safety trial
Oct 14, 2016 to May 31, 2017
HIGHLIGHTS

- Newly developed MRI techniques to track APCbased vaccines in the human body
- First time in Canada that the fate of injected therapeutic immune cells will be tracked in humans
About the project
Recently, immune-based therapies have been developed that control cancer progression. One approach is to target tumours with a tumour specific protein (antigen) vaccine. Immune cells, known as antigen presenting cells (APC), when loaded with a tumour antigen, activate other immune cells to specifically kill tumour cells. Cellbased cancer vaccine immunotherapy (CCVI) has shown potential for improvements in markers of tumour progression, quality of life and survival of treated patients. Yet CCVI has not met expectations of progression free survival, a goal that remains an unmet medical need. Thus, improvements to cancer vaccines are needed including the optimization of APC-based vaccine delivery to the immune system.
An important method now possible with MRI is the non-invasive tracking of transplanted therapeutic cells in patients undergoing treatment. In an immunotherapy setting, tracking infused optimized or engineered immune cells will confirm they localize to their intended target site, inform quantitatively on their number, persistence and viability thereby providing biomarkers predictive of therapeutic outcome in real time.
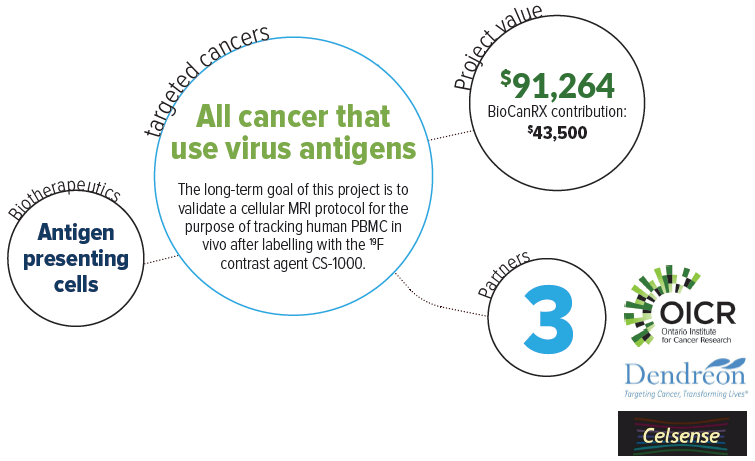
To do this, the team developed magnetic resonance imaging (MRI) techniques to track APC-based vaccines in the human body. This will determine if the APC-based vaccine reaches immune tissues and the tumour, whether sufficient numbers of the vaccine APC are present to stimulate successful tumour cell killing and how long the vaccine APC persist in a cancer patient. To distinguish cancer vaccine APC from the surrounding tissue the APC are labeled with a contrast agent. The contrast agent creates a MRI detectable signal wherever these labeled cells are located. It remains to be proven whether injected therapeutic cells actually reach their intended target (lymphoid organs or tumour) in ample numbers to create an effective anti-tumour response.
This project will determine the feasibility and safety of using perfluorocarbon nanoemulsion (Cell Sense [CS-1000]) as a contrast agent to assess delivery of APC-based vaccines in humans. This will be the first time in Canada that the fate of injected therapeutic immune cells will be tracked in humans, a technology that is applicable to the treatment of many cancers. Providing a diagnostic biomarker will allow physicians to make real time assessments of therapy and adjust patient treatment accordingly.