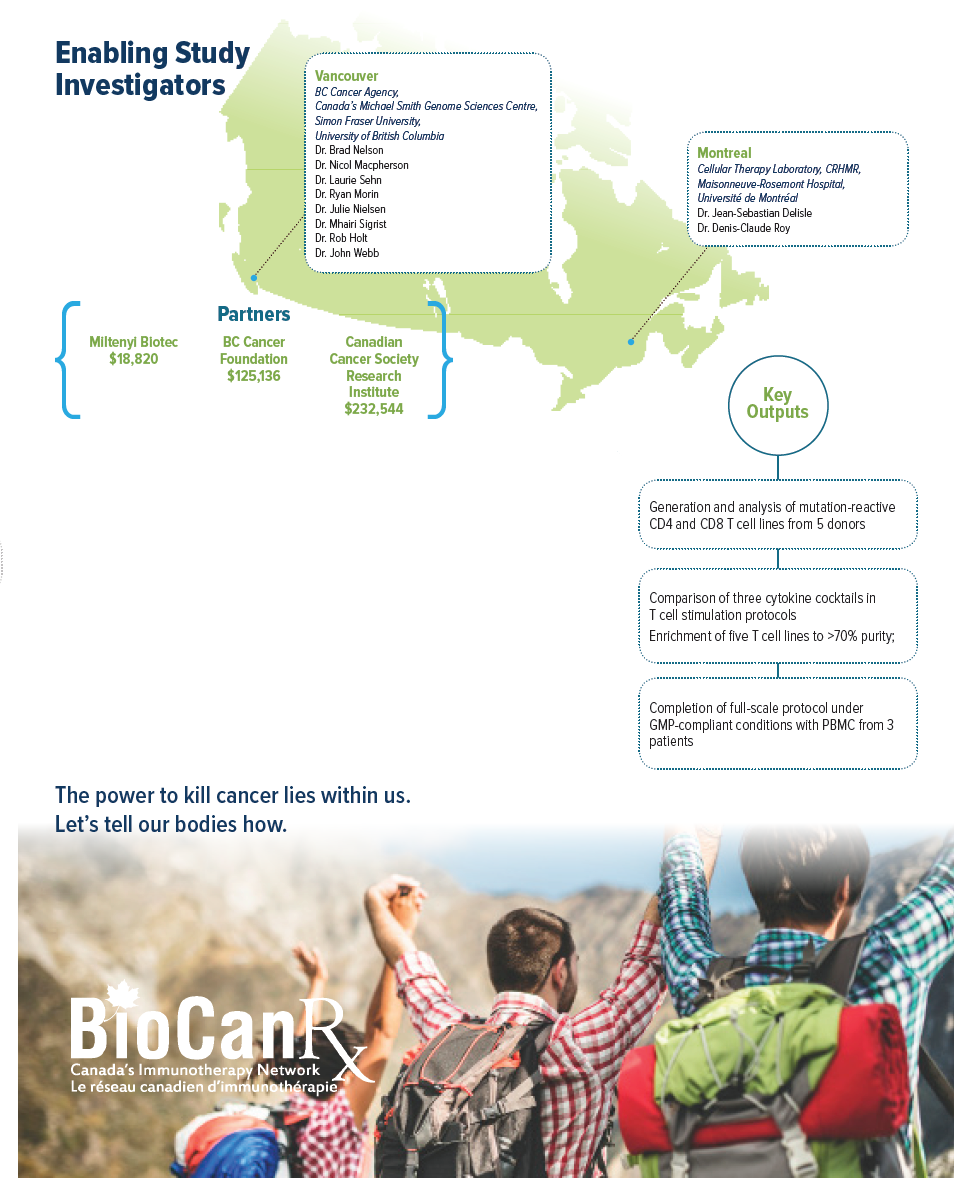
Project summary: Enabling Studies Program
Adoptive T0cell therapy targeting patient-specific driver mutations in lymphoma
Oct 14, 2016 to Nov 30, 2019
HIGHLIGHTS

- Strategy to treat tumors using mutation-specific T cells
- Subsequent clinical testing will determine whether the infusion of billions of mutation-specific T cells into the bloodstream of lymphoma patients is safe, feasible, and effective
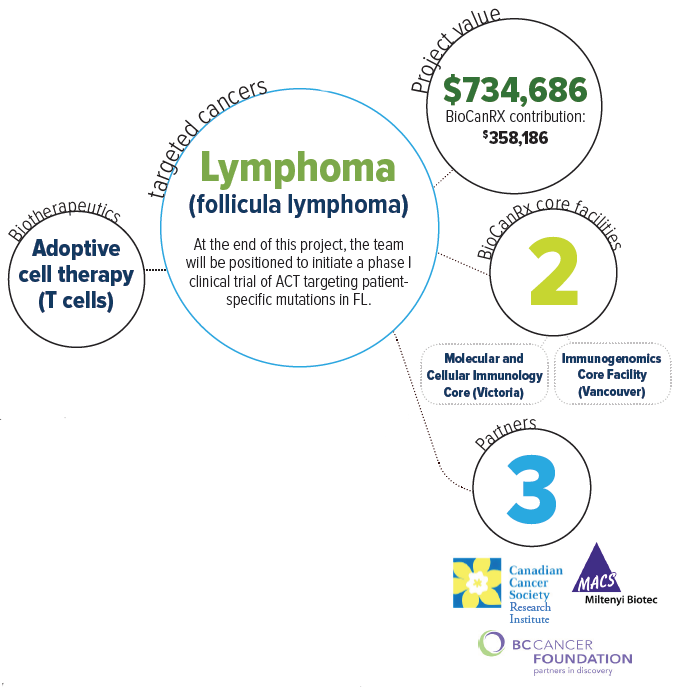
About this project
Many types of lymphoma are incurable with current therapies; therefore, new treatments are urgently needed. The immune system plays a major role in controlling cancer, and several new immune based therapies have recently produced groundbreaking clinical responses. An important challenge in developing new therapies is to find targets that are unique to cancer cells so that one can launch a potent immune response without causing unacceptable side effects. The mutations found in tumors represent ideal targets, especially those that are important for cancer survival (socalled “driver” mutations). In lymphoma, about 50 genes have been found to frequently contain mutations, providing a shortlist of target antigens. Moreover, the team recently showed that many lymphoma patients have T cells in their blood that can detect driver mutations and attack cancer cells. This opens the possibility of using mutationspecific T cells to treat lymphoma and other cancers.
It is now feasible to identify the entire repertoire of mutations in a patient’s tumor; however, many mutations remain difficult to target pharmacologically. As an alternative, this project proposes to treat tumors using mutation-specific T cells. In support of this concept, the team recently reported that T-cell responses against common driver mutations can be identified in patients with follicular lymphoma (FL).
Mutation-specific T cells underlie clinical responses to immune-based therapies such as immune checkpoint blockade and adoptive cell therapy (ACT). ACT is a promising therapeutic approach that has been tested in several settings, from preventing virus reactivation after stem cell transplant to treating metastatic melanoma. While CD8+ T cells are often considered the key mediators during ACT, mutation-specific CD4+ T cells are also therapeutically important in murine models and patients.
Follicular lymphoma (FL) – the most common indolent non-Hodgkin lymphoma – is incurable with standard therapies. However, evidence suggests that it is immunologically sensitive. For example, TIL are associated with increased patient survival, and up to 30% of patients experience spontaneous remissions. Moreover, clinical benefit has been observed in a subset of FL patients vaccinated against tumor-specific immunoglobulins or treated with immune checkpoint blockade. While encouraging, these results highlight the need to develop strategies to achieve higher response rates.
This project endeavors to define the optimal methods to expand and enrich mutation specific T cells in the lab and perform validation experiments in preparation for a phase I clinical trial involving 10 lymphoma patients. Their overarching goal is to determine whether the infusion of billions of mutationspecific T cells into the bloodstream of lymphoma patients is safe, feasible, and potentially effective. In the future, this approach can be applied to additional patients, including those with other types of cancer, resulting in even broader clinical impact. This work will help lead the way to more potent and precise immunotherapies for cancer.
This project proposes to develop an immunotherapy for FL that is more effective than vaccination while causing less toxicity than CAR T cells. FL offers the experimental advantage of having a well-defined set of driver mutations that are frequently shared between patients. Indeed, by sequencing only 10 genes that frequently harbor driver mutations, the team identified mutations in 81% of cases. Although they did not experimentally confirm that these were driver mutations, the most common mutations they found are well established drivers. Using in vitro stimulation of autologous peripheral blood mononuclear cells (PBMC), they identified CD8+ T cells specific for known or putative drivers in 23% of tested patients. These T cells were present at very low levels in PBMC, highlighting both the need and the opportunity to amplify these responses to therapeutic levels.
At the conclusion of this project, the team anticipate being positioned to initiate a phase I clinical trial.