Project summary: Enabling Studies Program
DEVELOPMENT OF IMMUNE REGULATING ANTIBODIES FOR USE IN COMPANION ANIMAL CLINICAL TRIALS
July 1, 2015 to March 31, 2020
HIGHLIGHTS

- An innovative approach to accelerating combination cancer therapies by using companion animal clinical trials
- Companion animals are cancer patients themselves and could benefit from clinical application of novel experimental therapeutics
- This project will develop canine-active synthetic antibodies that bind to key immune modulatory molecules in development for use in human cancer patients
- This project will test the ability to rapidly assess combinations of antibodies with other innovative therapies (e.g., oncolytic viruses, T cells) in a clinical setting
- Cost effective product development
ABOUT THE PROJECT
Many novel biotherapies (e.g., synthetic antibodies and oncolytic viruses) are currently being developed for cancer treatment. However, trying to unravel the best combination strategies to develop for clinical trials poses a daunting challenge and is not economically feasible or practical in the current regulatory setting. While mouse tumour models allow good assessment of the activity of drugs on biological processes in the body, they do not accurately reflect the human situation.
This project proposes to accelerate the testing and optimization of biotherapeutic strategies by using companion animals that spontaneously develop tumours late in life, in the context of a normal, outbred immune system. This cancer development parallels human cancer development. The project’s proposed testing will provide a bridge between academic discoveries validated in mouse models and human clinical trials.
As a proof-of-concept, we will test single biotherapies of optimized antibody drug candidates, with the ultimate goal of providing a platform whereby biological drugs developed within the BioCanRx network can be rapidly tested in veterinary clinical trials in order to shortlist the most promising candidates and combinations for translation into human patients.
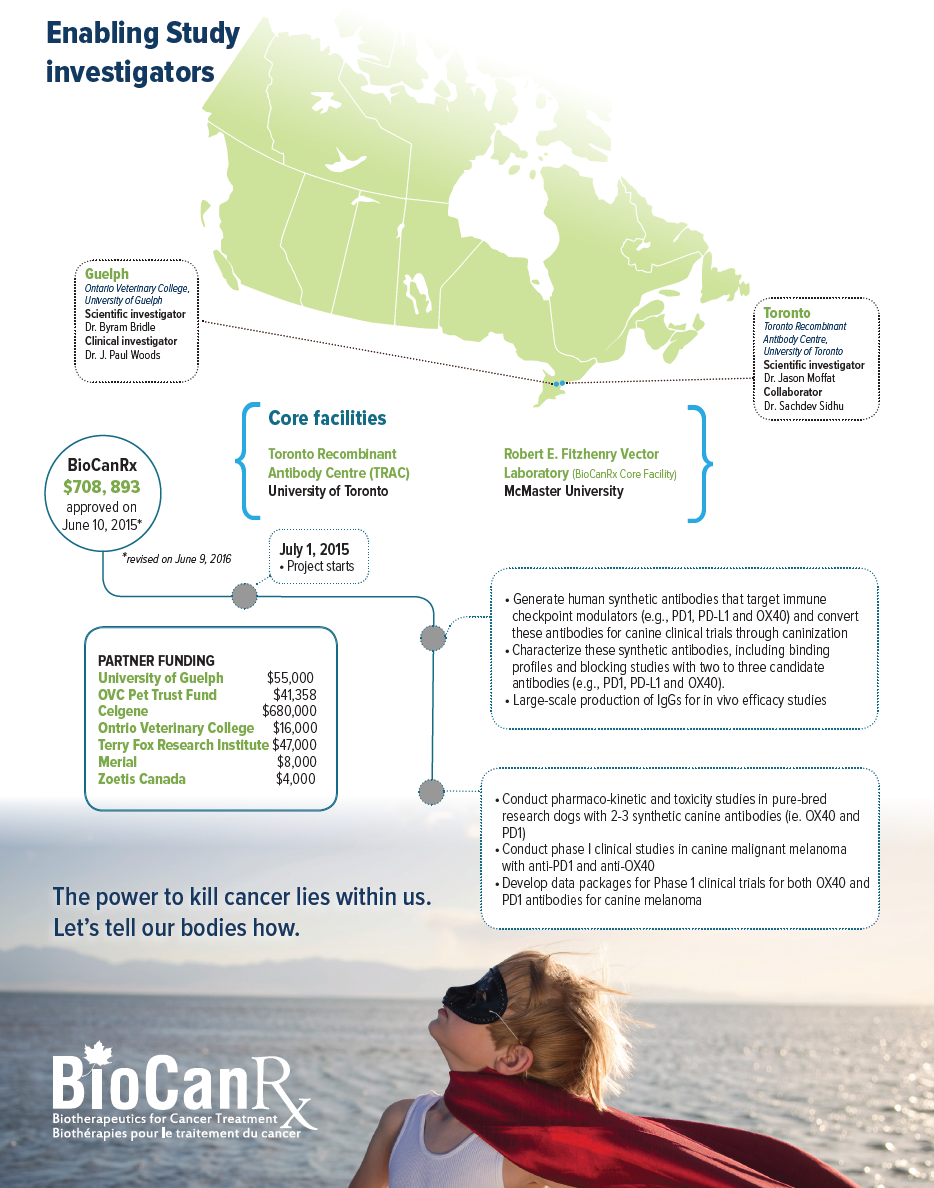
SCIENTIFIC INVESTIGATORS
- Dr. Jason Moffat, Toronto Recombinant Antibody Centre, University of Toronto
- Dr. Byram Bridle, Ontario Veterinary College, University of Guelph
CLINICAL ADVISORS
- Dr. J. Paul Woods, Ontario Veterinary College, University of Guelph
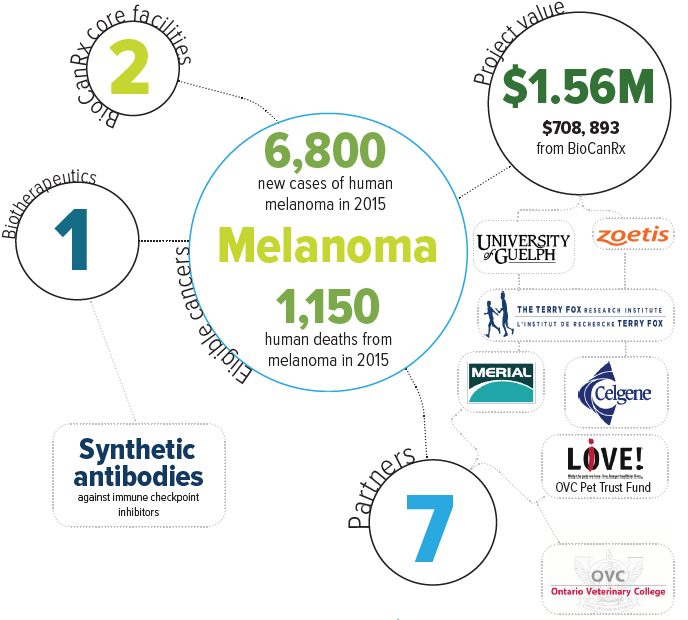
Keywords: synthetic antibodies, immune checkpoint inhibitors
Eligible cancers: melanoma
Partners: Celgene

