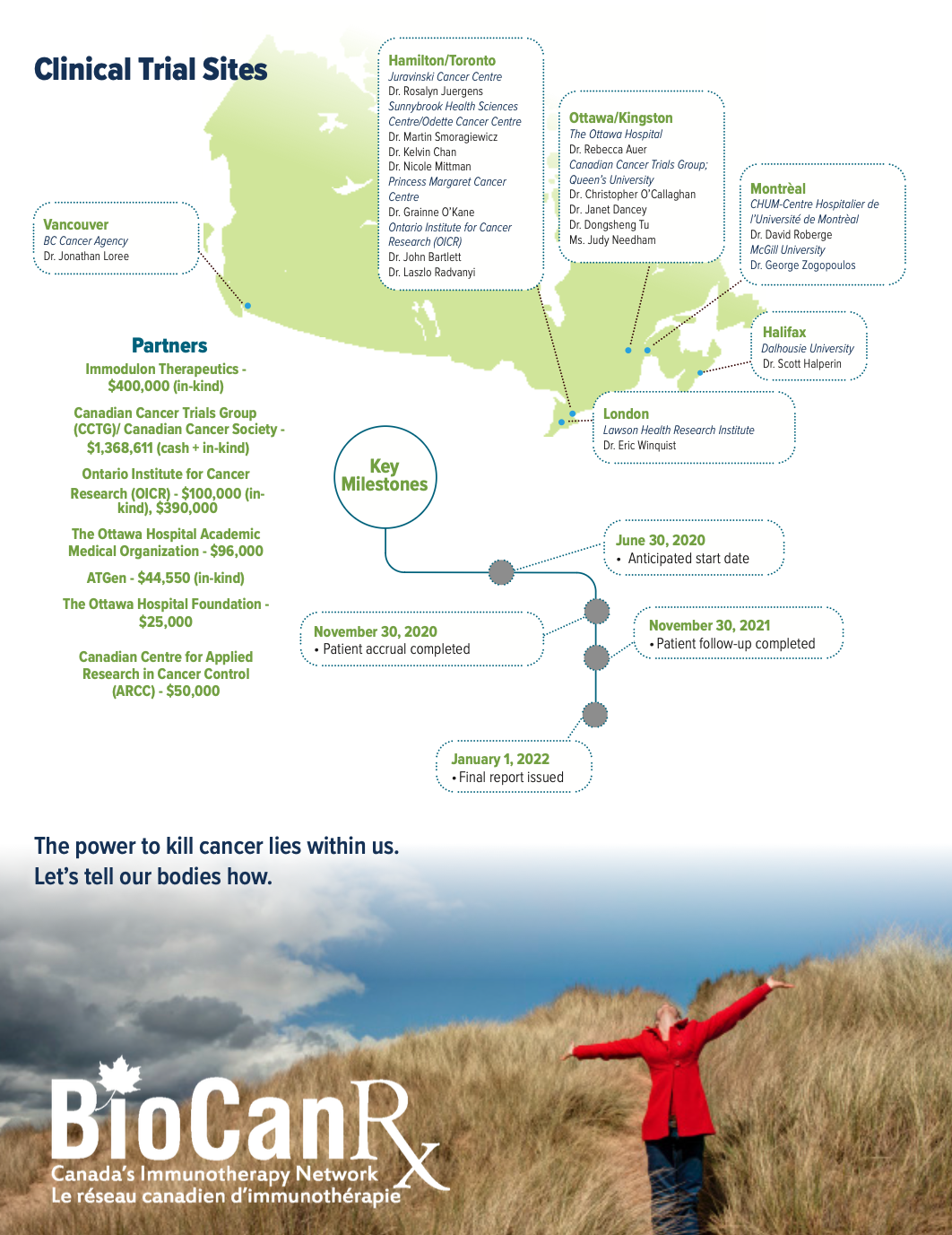
Project summary: Clinical Trial
COV-IMMUNO- A randomized phase III trial of immunization with IMM-101 versus observation for the prevention of serious respiratory and COVID-19 related infections in cancer patients at increased risk of exposure
April 1st, 2020 to December 31, 2022
HIGHLIGHTS

- This trial has been developed to address a critical and urgent need to protect cancer patients undergoing active treatment during the SARS-COV2 pandemic.
- Cancer patients are particularly vulnerable to severe COVID-19 infections because they are both immunocompromised and cannot adhere to strict quarantine as they need to visit the hospital regularly for treatment.
- IMM-101 is a safe, killed, whole cell immunomodulator that has been shown to induce an innate immune response in cancer patients.
- Cancer patients are particularly vulnerable to severe COVID-19 infections because they are both immunocompromised and cannot adhere to strict quarantine as they need to visit the hospital regularly for treatment.
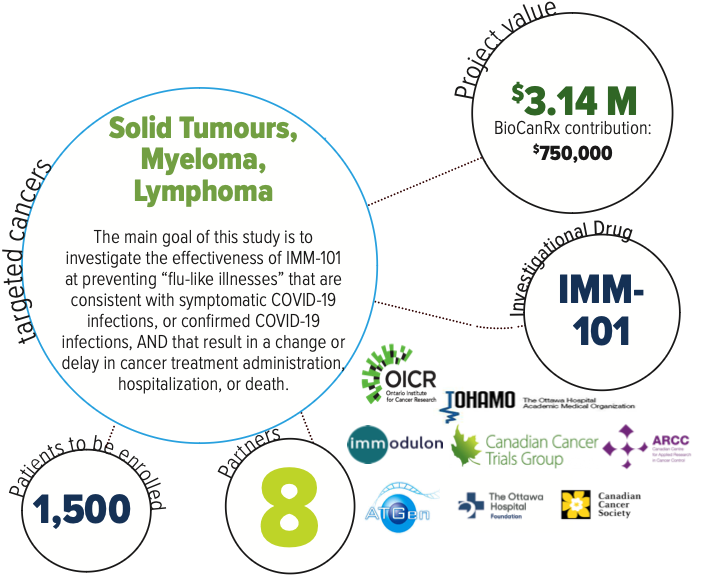
About the Project
As cancer patients have dysfunctional innate immune responses, they are at higher risk of severe COVID-19 infections, which at best, can result in delays in their treatment of their active cancer, and at worst, an increased incidence of mortality. While numerous efforts are currently underway globally to design and test a safe and effective COVID-19 vaccine that provides long-term and repeated protection, the timeline for its wide-spread availability is approximately 12-18 months.
Stimulation of the innate immune system (known as “trained immunity”) is a promising approach to optimizing the innate to adaptive transition for many infections, including COVID-19. This principle has been shown in the past with recipients of the tuberculosis vaccine, known as BCG, demonstrating an increased resistance to multiple other infections due to a parallel, non-specific stimulation of their innate immunity. While BCG vaccination is being tested in healthcare workeres against COVID-19 infection in multiple clinical trials around the globe, because it is composed of a live (but modified) bacteria, its use is contraindicated in patients with a weakened immune system, such as cancer patients.
In contrast, IMM-101, the investigational drug for this trial, is a whole cell immunomodulator that is safe to use in cancer patients because the bacteria has been killed. IMM-101 (Immodulon Therapeutics) is a systemic immune modulator containing a suspension of heat-killed whole cell Mycobacterium obuense, an environmental, harmless saprophyte. Given that it is NOT a live vaccine, it is being developed as an anti-cancer therapy, based on the same rationale of trained immunity, but against cancer cells. IMM-101 has been shown to induce an innate immune response in cancer patients of equal or greater magnitude to that reported with BCG treatment.
While this trial is not testing immunotherapy for the treatment of cancer, it has the potential to not only lessen respiratory symptoms for cancer patient such as those that present because of COVID-19, enabling patients to be physically able to receive active cancer therapy, but also reduce the incidence of mortality due to respiratory illnesses such as COVID-19.