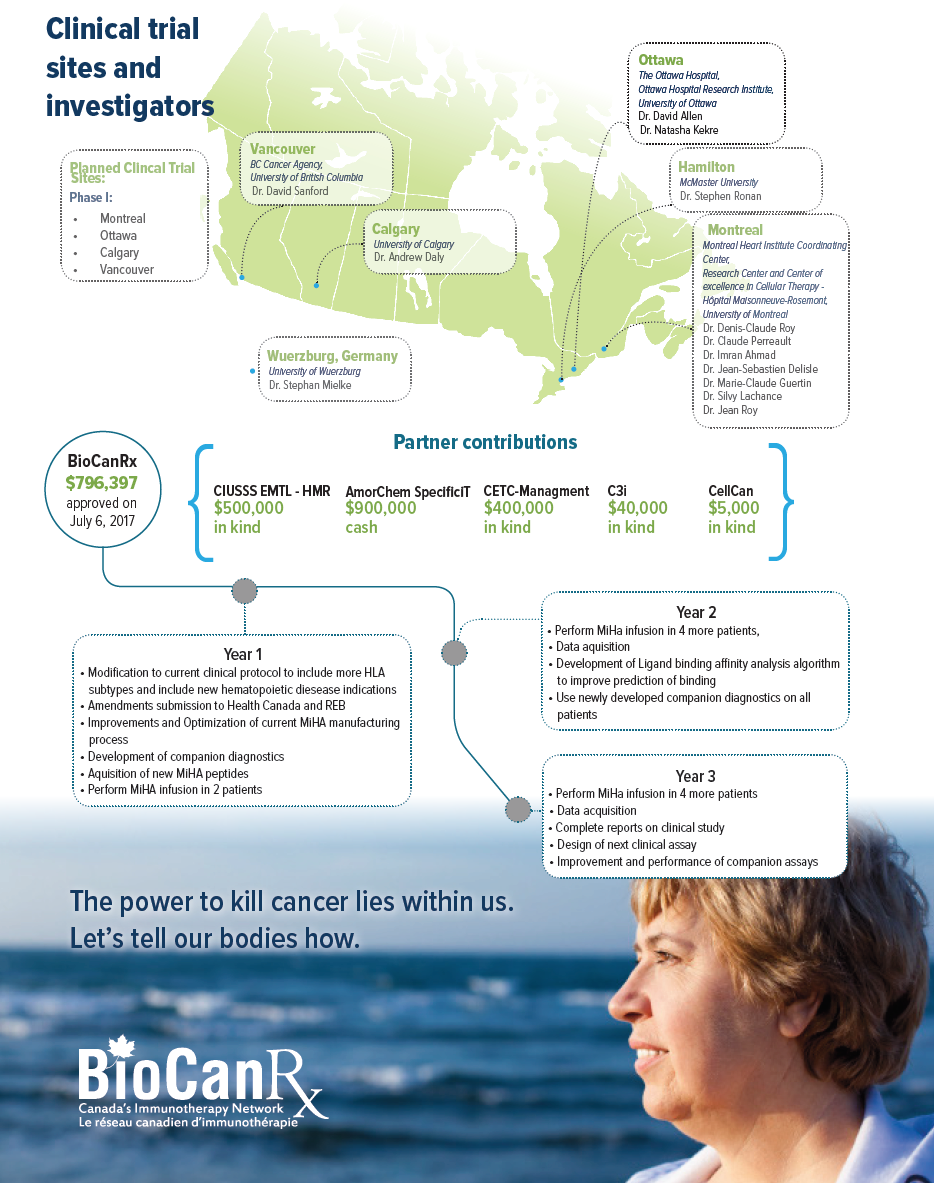
Project summary: Clinical Trials Program
Phase I clinical trial of Anti-Minor Histocompatibility Antigen Immunotherapy Broadening the Scope of Application for Precision Therapeutics
July 1, 2017 to September 30, 2021
HIGHLIGHTS

- First internationally to identify so many relevant and validated MiHAs and generate T-cell lines against these MiHAs.
- A novel strategy to enhance the Graft-Versus-Tumor Effect (GVTE) and circumvent GVHD of AHCT.
- Adoption of MiHAtargeted immunotherapy will allow for safe, targeted and more effective treatment of patients with HCs without increasing the global cost of HC
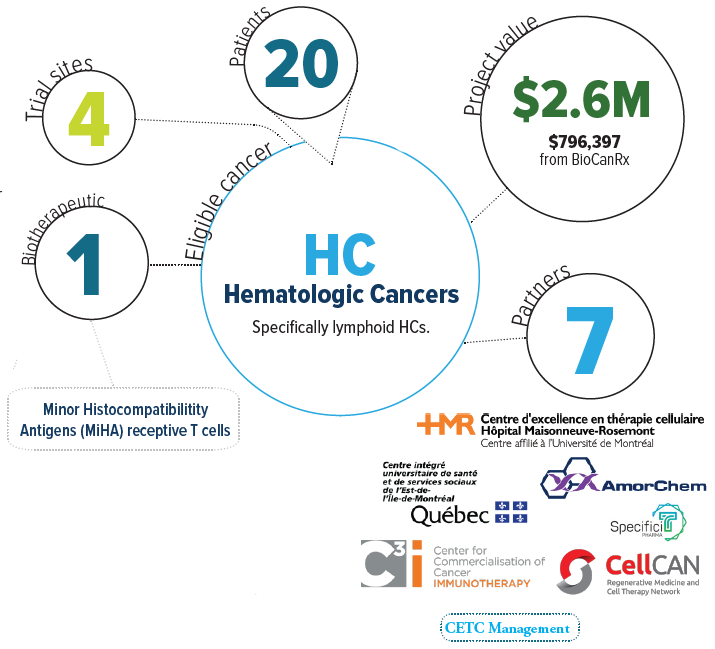
About the project
Hematologic cancers (HCs), i.e. cancers that effect the blood and lymph system, represent about 10% of all cancers. The number of new cases of HCs in Canada is 16,000 per year. HC affects both adults and children, and while around 50% of patients with HC can be cured by chemotherapy, the other 50% develop resistance to chemotherapy and die. This project’s objective is to provide safer and more effective treatments for patients with resistant HCs. For most of these patients, allogeneic hematopoietic cell transplantation (AHCT) is the only curative treatment. It is now known that the curative effects of AHCT results from immune system cells that recognize or target tumor Minor Histocompatibility Antigens (MiHAs), small cell-surface proteins that function as ‘signals’ for immune system cells. However, the use of adoptive immunotherapy is hampered by two factors: i) the variable anti-HC activity of AHCT, and ii) the risk of a devastating complication, graft-vs.-host disease (GVHD=donor cells attacking the patient). Currently, the inability to selectively target malignant cells leads to the occurrence of GVHD. The team envisions that their work will transform AHCT into a consummate model of personalized cancer therapy because of their strategy will wallow to tailor AHCT components as a function of the proteome of the cancer cells. They are initiating a phase I clinical trial to test this novel immunotherapeutic strategy in patients. The T cell product with anti-MiHA activity has been called “GLIDE” for Guided Lymphocyte Immunotpeptide Derived Expansion against MiHAs.
The team has identified 98 MiHAs that have preferential expression on hematopoietic cells, thus minimizing the risk of GVHD. Also, they were able to develop an ex vivo immunization strategy that allows to generate T cells with anti-MiHA specificity. They will initiate a clinical trial using this novel strategy to treat patients with lymphoid HCs and also expand the HLA typing repertoire in order to increase patient reach to approximately 95% of the patient population (from the current 45% reach). MiHA-targeted immunotherapy would allow for safe, targeted and more effective treatment of patients with otherwise fatal HCs.