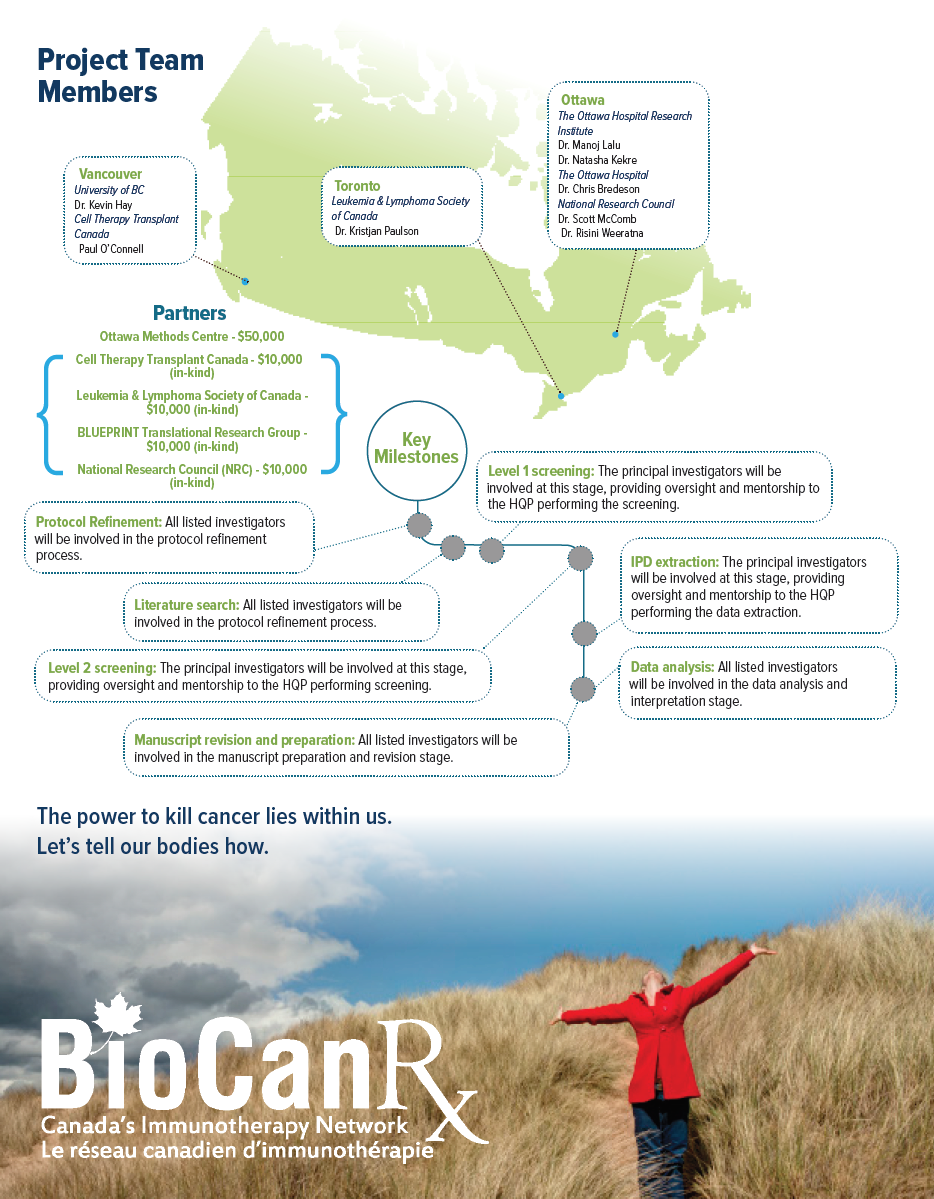
Project summary: Clinical, Social and Economic Impact
Identifying Effect Modifiers of CAR T-Cell Therapeutic Efficacy
July 1, 2020 – October 31, 2022
HIGHLIGHTS

- The heterogeneity seen in efficacy both between and within clinical trials has been a detriment to the advancement of the field of CAR T-cell therapy.
- Using individual patient data, as opposed to data aggregated at the study level, improves the ability to identify important characteristics which may be impacting the efficacy of CAR T-cell therapy.
- Individual patient data metaanalyses have demonstrated the ability to help shape and inform clinical trial design, an important and relevant feature in the fast-moving field of CAR T-cell therapy.
- Given the safety concerns and the cost currently surrounding CAR T therapy, it is important that the right group of patients be properly identified and treated safely.
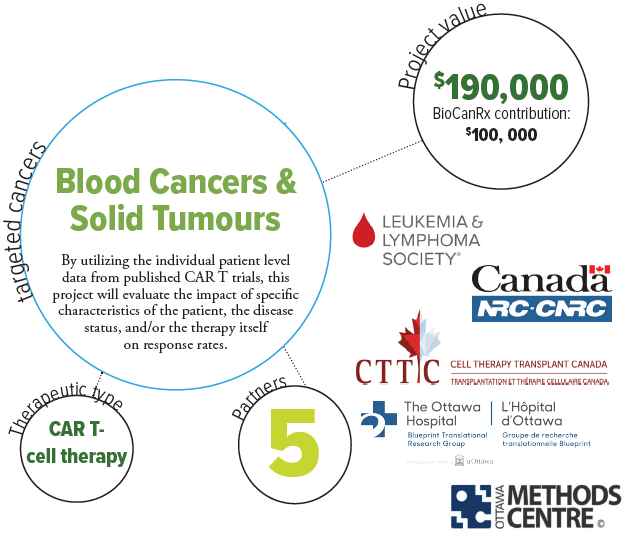
About the Project
Chimeric antigen receptor T-cell therapy is a promising new treatment option for patients with relapsed or refractory blood cancers. Although small clinical trials of CAR T-cells have been conducted and demonstrated exciting results, potential issues with safety, efficacy, and economic viability have been identified. The researchers’ recent review of published CAR T-cell trials (funded by BioCanRx) demonstrated that the therapy can be extremely effective in some people and not others. The reasons for this difference remain unclear but emerging evidence suggests that specific characteristics about either the people (for example biological sex or age), disease status or of the therapy itself (dose, for example), can lead to differences in efficacy between patients.
By utilizing the individual patient level data from each of the published trials, this project will evaluate the impact of these characteristics on response rates. This powerful approach is known as an individual patient data systematic review and metaanalysis. At the same time, the project will provide an updated review of all CAR T-cell trials in blood and solid tumor cancer patients. This will help clinicians and decision-makers optimize CAR T-cell therapy for patients, and help inform the design of future clinical trials and interventions that may demonstrate better efficacy. Identifying characteristics that may act as modifiers of CAR T-cell efficacy is of paramount importance and can help shape future clinical trials in the field. Results from this project have the potential to not only impact future BioCanRx projects, but CAR T-cell projects around the world. The ultimate goal is to create better and safer outcomes for patients undergoing CAR T-cell therapy.