Project summary: Clinical, Social and Economic Impact Program
IMPROVING THE QUALITY OF JUDGEMENT IN CANCER THERAPEUTICS DEVELOPMENT
April 1, 2016 to June 30, 2019
HIGHLIGHTS

- Provide cancer researchers with critical tools to improve decision-making surrounding research, development and study design;
- Establish the feasibility of improving researcher judgement in drug development
- Measure the value of improving researcher judgement, minimizing patient burden and maximizing the efficient use of public investments in drug development.
ABOUT THE PROJECT
Therapeutic cancer drug development requires good judgment about product safety, efficacy and the feasibility of trials. However, we know next to nothing about the quality of this judgment. What we do know is that most cancer therapies put into development either fail to show benefit or show unexpected toxicity; that research often takes longer than expected; and that many trials fail to recruit enough patients. We also know that experts in medicine, science and innovation are prone to ignoring conflicting evidence, and overestimating the benefits and feasibility of their projects. Consequently, there is good reason to suspect that expert judgment in the development of cancer therapies could be improved.
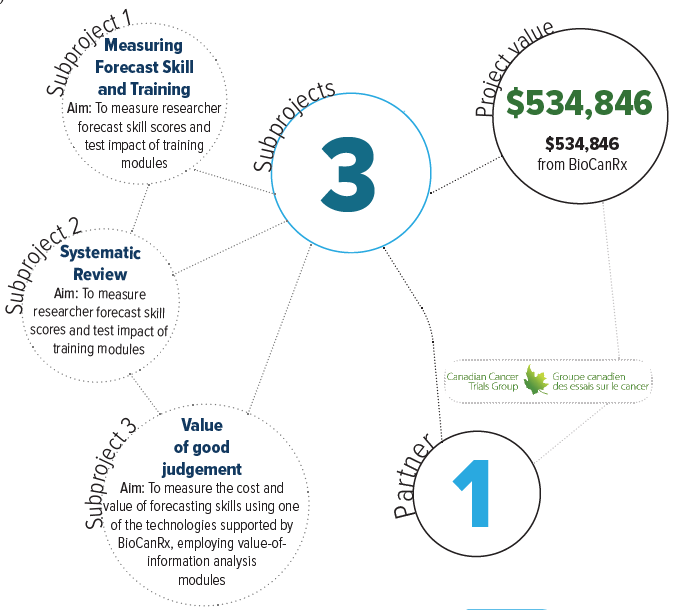
Through three subprojects, Dr. Kimmelman’s team will directly measure and refine the quality of judgements from a wide array of experts at various critical times in the development process of several types of cancer biotherapeutics. This data will provide BioCanRx with the ability to interpret systematic reviews, and apply better scientific judgement in relation to projects within the network.
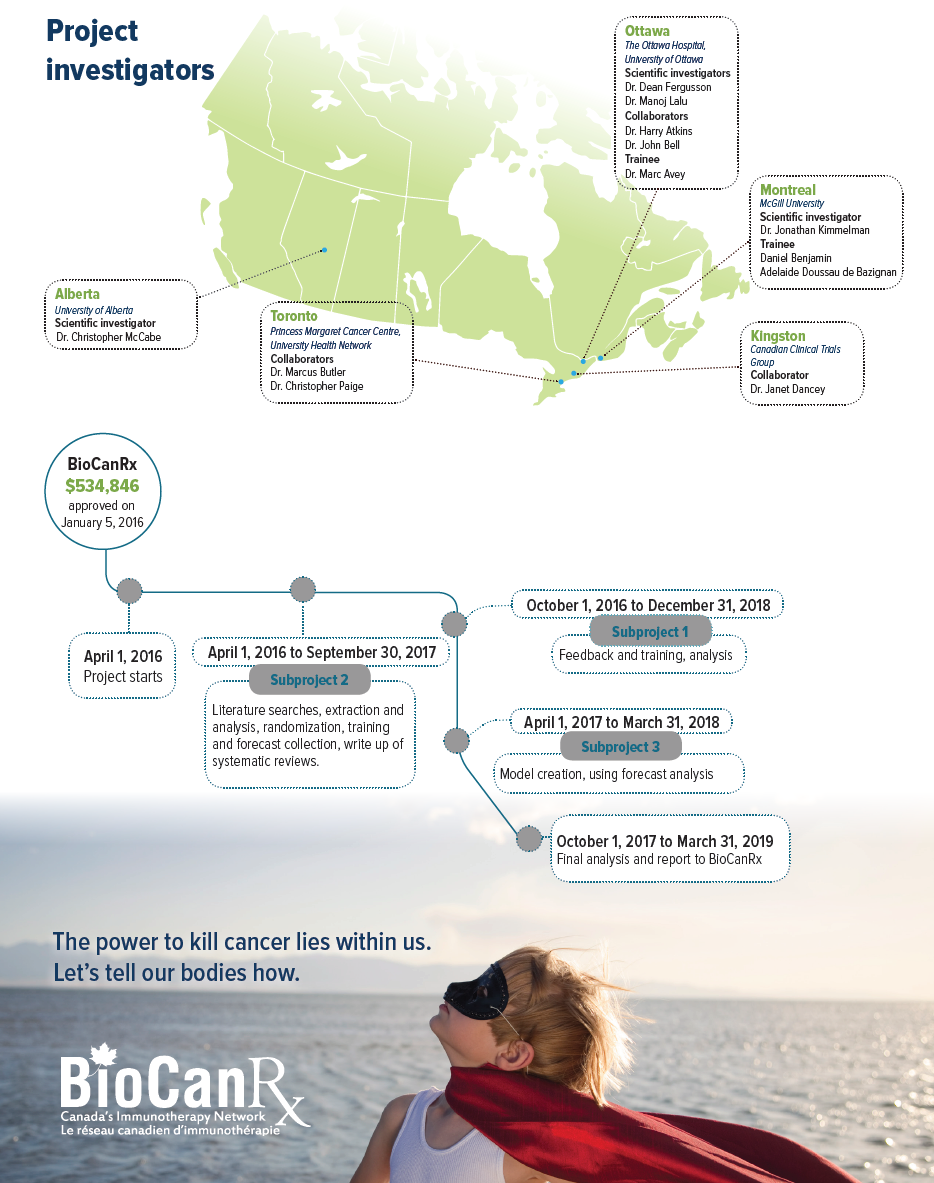
SCIENTIFIC INVESTIGATORS
- Dr. Dean Fergusson, The Ottawa Hospital, University of Ottawa
- Dr. Jonathan Kimmelman, McGill University
- Dr. Manoj Lalu, The Ottawa Hospital, University of Ottawa
- Dr. Christopher McCabe, University of Alberta
COLLABORATORS
- Dr. Harry Atkins, The Ottawa Hospital, University of Ottawa
- Dr. John Bell, The Ottawa Hospital, University of Ottawa
- Dr. Marcus Butler, Princess Margaret Cancer Centre, University Health Network
- Dr. Janet Dancey, Canadian Clinical Trials Group
- Dr. Christopher Paige, Princess Margaret Cancer Centre, University Health Network
TRAINEES
- Dr. Marc Avey, The Ottawa Hospital, University of Ottawa
- Daniel Benjamin, McGill University
- Adelaide Doussau de Bazignan, McGill University
Partners: Canadian Clinical Trials Group

