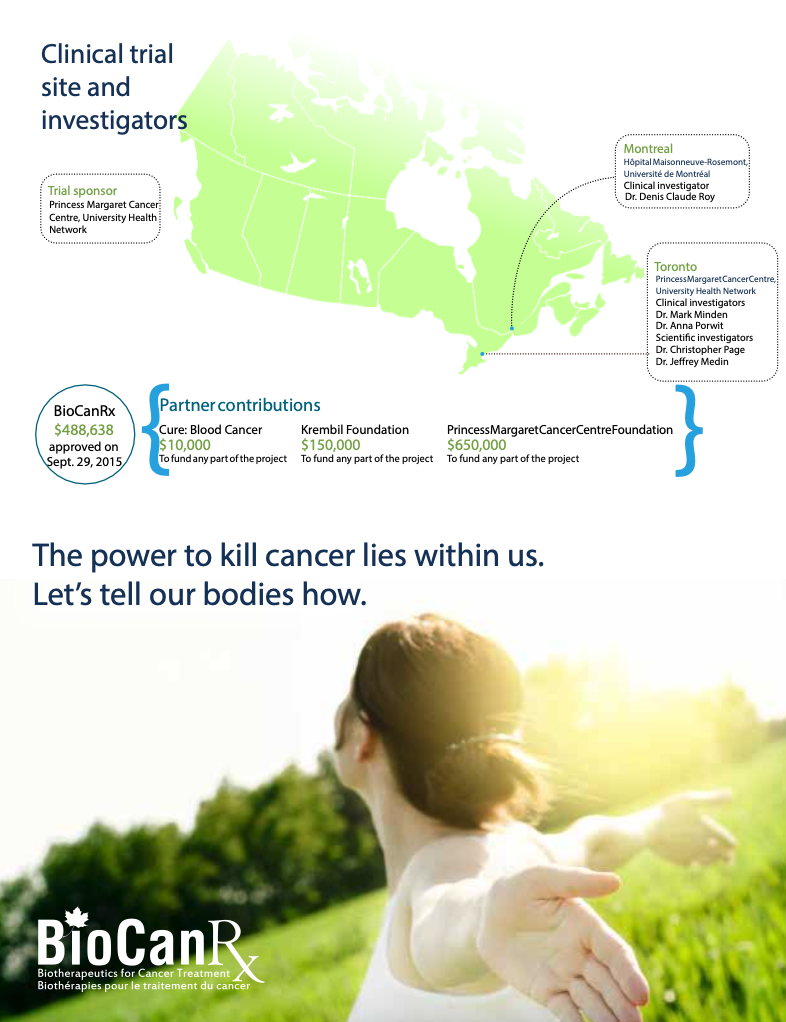
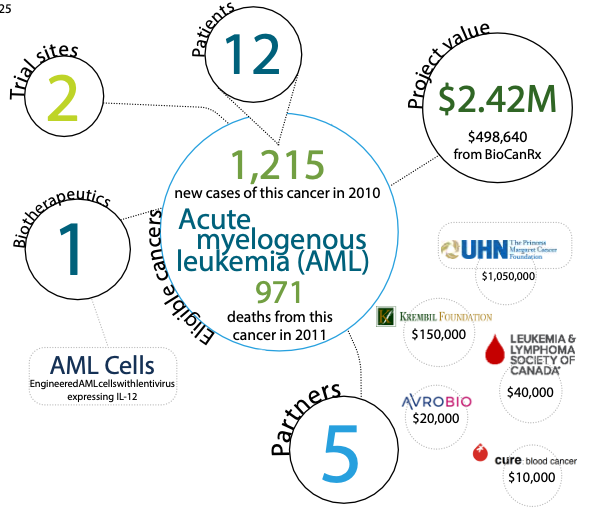
Project summary: Clinical Trials Program
Phase I study of therapy for acute myelogenous leukemia (AML) using autologous AML cells engineered to express IL-12
April 1, 2016 to March 31, 2023
HIGHLIGHTS

- This therapeutic approach has the potential to dramatically improve care for patients with relapsed AML through better outcomes and highly reduced toxicity risk, compared to current treatments.
- This approach overcomes many of the challenges associated with current treatment using matched donor bone marrow
- The systemic nature of this therapy can eradicate widespread disease.
- This approach can result in long-term immunological memory that is trained to recognize and attack cancer stem cells, by using a patient’s own cancer cells as a vaccine platform.
ABOUT THE PROJECT
The immune system has the capacity to kill leukemia cells if properly instructed to do so. Some of the key instructions come in the form of soluble proteins that, if present in the right amounts, help immune system cells recognize leukemia cells and become activated to kill them.
The project team has previously shown that leukemia cells can be modified to secrete one of these proteins, called Interleukin 12, or IL-12. In experimental systems the leukemia cells secreting IL-12 stimulated a robust immune response that, once initiated, went on to kill all the residual leukemia cells even those not secreting IL-12. Acute myeloid leukemia (AML) is a life-threating disease for which, in many cases, there is no curative treatment. This project will test the safety of infusing 10 to 12 patients with some of their own AML cells that have been engineered to secrete IL-12.
The clinical trial will determine if an immune response has been initiated in both the patient’s blood and bone marrow as treatment proceeds. It will also monitor the treatment’s effect on the level of disease and follow each patient for two years.