Project summary: Clinical Trials Program
EVALUATING ADOPTIVE CELL THERAPY TO TREAT OVARIAN CANCER USING TILS CONDITIONED WITH DENDRITIC CELLS
July 1, 2015 to April 30, 2018
HIGHLIGHTS

- World’s first Phase I clinical trial to use adoptive cell therapy for ovarian cancer.
- World’s first clinical test of a Canadian innovation to re-activate TILs derived from a patient’s tumour.
- First use of a TIL cancer therapy in Canada, imported by a world-renowned thought leader in tumour immunology.
- Fosters the spread of this promising TIL platform to other clinical sites in Canada.
ABOUT THE PROJECT
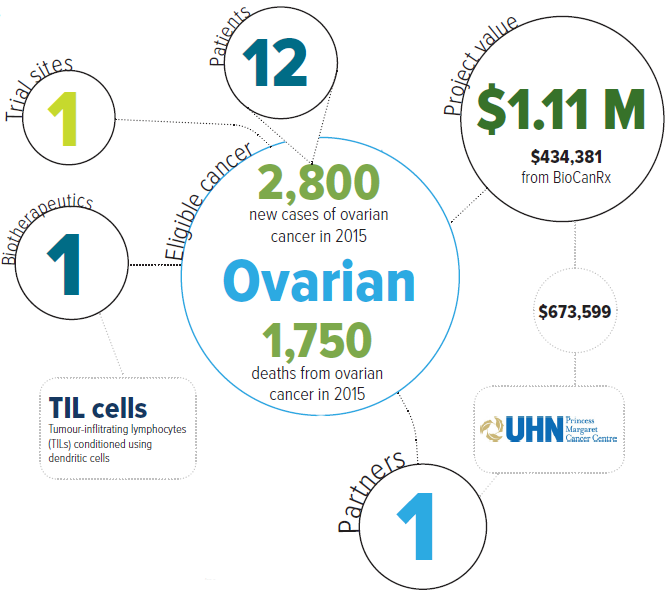
Ovarian cancer is the fifth most deadly cancer in women and the most lethal gynecologic malignancy. Surgery and chemotherapy are currently the standard treatments, but the death rate for this cancer remains very high. This trial brings an innovative biotherapeutic approach that uses a population of cells from the immune system, called T cells, to treat this deadly disease.
T cells are white blood cells that have the ability to seek out and destroy tumours. The T cells found within a tumour are called tumour-infiltrating lymphocytes, or TILs. Known as adoptive cell therapy, this approach to cancer treatment takes a patient’s own TILs and re-activates them in the laboratory before giving them back to the patient. This therapy has been tested in patients with metastatic melanoma and shows promise, serving as a catalyst to test it in ovarian cancer.
The team at UHN’s Princess Margaret Cancer Centre has developed a novel method to increase the activity of TILs in the laboratory. As a result, this project will be a first-in-human clinical study on two fronts. It will evaluate this innovative method of activating TILs and will also be the first to use TIL therapy for ovarian cancer, specifically platinum-resistant ovarian cancer.
This study aims to serve as a basis for further clinical development of this “made in Canada” approach to TIL therapy and to determine its potential effectiveness as a therapy for what has traditionally been a very difficult cancer to treat.
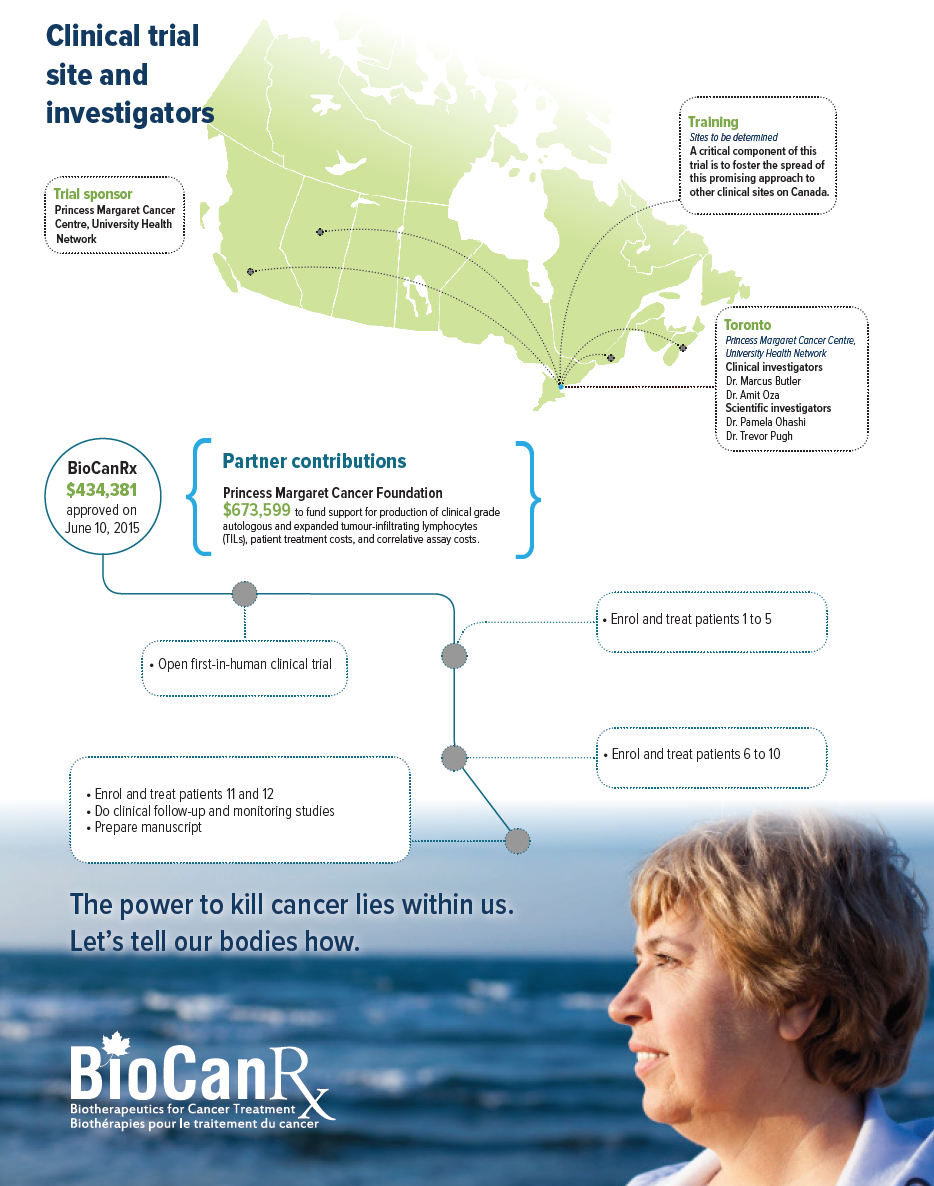
A key component of this project will be training investigators, from across Canada, how to they can perform TIL therapy at their hospitals.
TRIAL SPONSOR
- Princess Margaret Cancer Centre, University Health Network
SCIENTIFIC INVESTIGATORS
- Dr. Pamela Ohashi, Princess Margaret Cancer Centre, University Health Network
- Dr. Trevor Pugh, Princess Margaret Cancer Centre, University Health Network
CLINICAL ADVISORS
- Dr. Marcus Butler, Princess Margaret Cancer Centre, University Health Network
- Dr. Amit Oza, Princess Margaret Cancer Centre, University Health Network
Keywords: adoptive cell therapy, TIL cells, tumour-infiltrating lymphocytes, dendritic cells
Eligible cancers: ovarian
Partners: Princess Margaret Cancer Centre Foundation

