Project summary: Clinical Trial
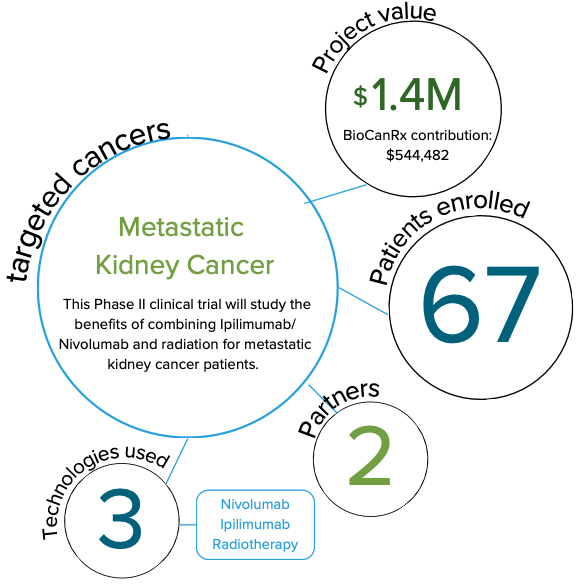
Phase II CYTOSHRINK Trial: Cytoreductive Stereotactic Hypofractionated Radiotherapy with Combination Ipilimumab and Nivolumab for Metastatic Kidney Cancer
April 1st, 2020 to March 31st, 2023
HIGHLIGHTS

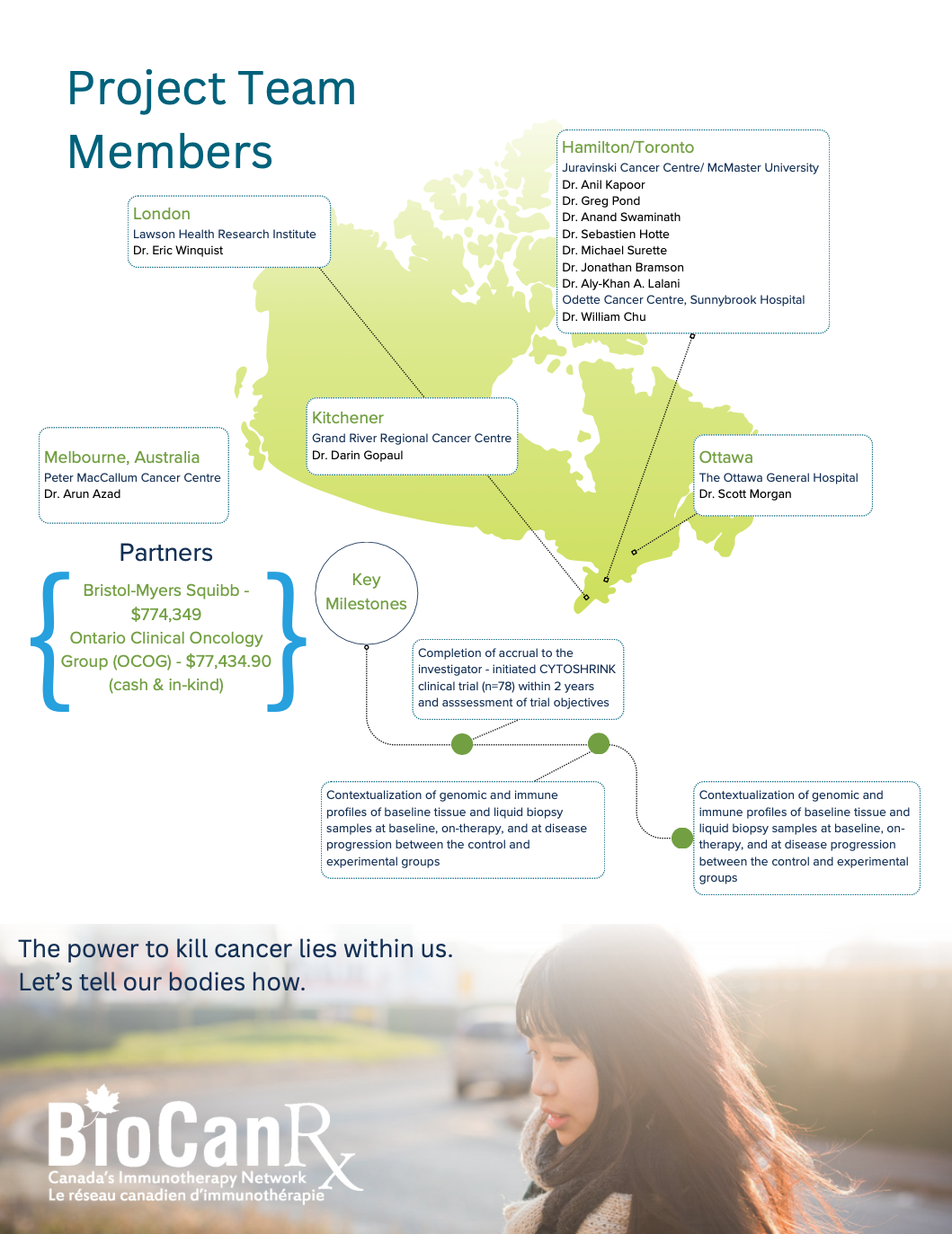
- The phase II, randomized CYTOSHRINK clinical trial is innovative and unique as it evaluates treatment with SBRT and immunotherapy for metastatic renal cell carcinoma (mRCC) across multiple Canadian sites.
- The interrogation of host samples of tumor tissue, blood and stool from immunological and genomic perspectives will provide a deeper understanding of tumor biology at the start and changes during treatment with this novel combination strategy.
- This proposal aligns with the overarching BioCanRx area of focus on combination immunotherapy strategies and emphasizes a multi-disciplinary collaboration in a deliverable Canadian context.
- This trial is the first to prospectively study Stereotactic Body Radiation Therapy (SBRT) directed at the primary lesion in a metastatic context combined with immunotherapy.
About the Project
Immune checkpoint blockers, such as Ipilimumab and Nivolumab, have been shown to improve the lifespan of some, not all, patients with metastatic kidney cancer. Understanding the factors that indicate which patients are likely to benefit from treatment is very important.
Removal of the primary kidney was shown over 20 years ago to be modestly beneficial for metastatic kidney cancer patients; however, recent studies cast doubt on this benefit in the current immunotherapy era. Alternatively, highly focused radiation is a convenient, safe method to kill cancer cells that may also enhance our immune response.
In this study, the researchers hypothesize that combining immunotherapy and highly focused radiation will improve the treatment of metastatic kidney cancer. To address this, they plan to study the benefits of combining Ipilimumab/Nivolumab and radiation for metastatic kidney cancer patients. Additional information will also be gathered on how patients respond to immunotherapy plus radiation by studying changes in their blood and gut bacteria during treatment.
In line with the BioCanRx mission, this will result in identification of non-invasive biomarkers for more precise patient selection when employing these combination modalities. Ultimately, improved combination therapies with better cancer control will significantly impact the quantity and quality of life for Canadian patients.