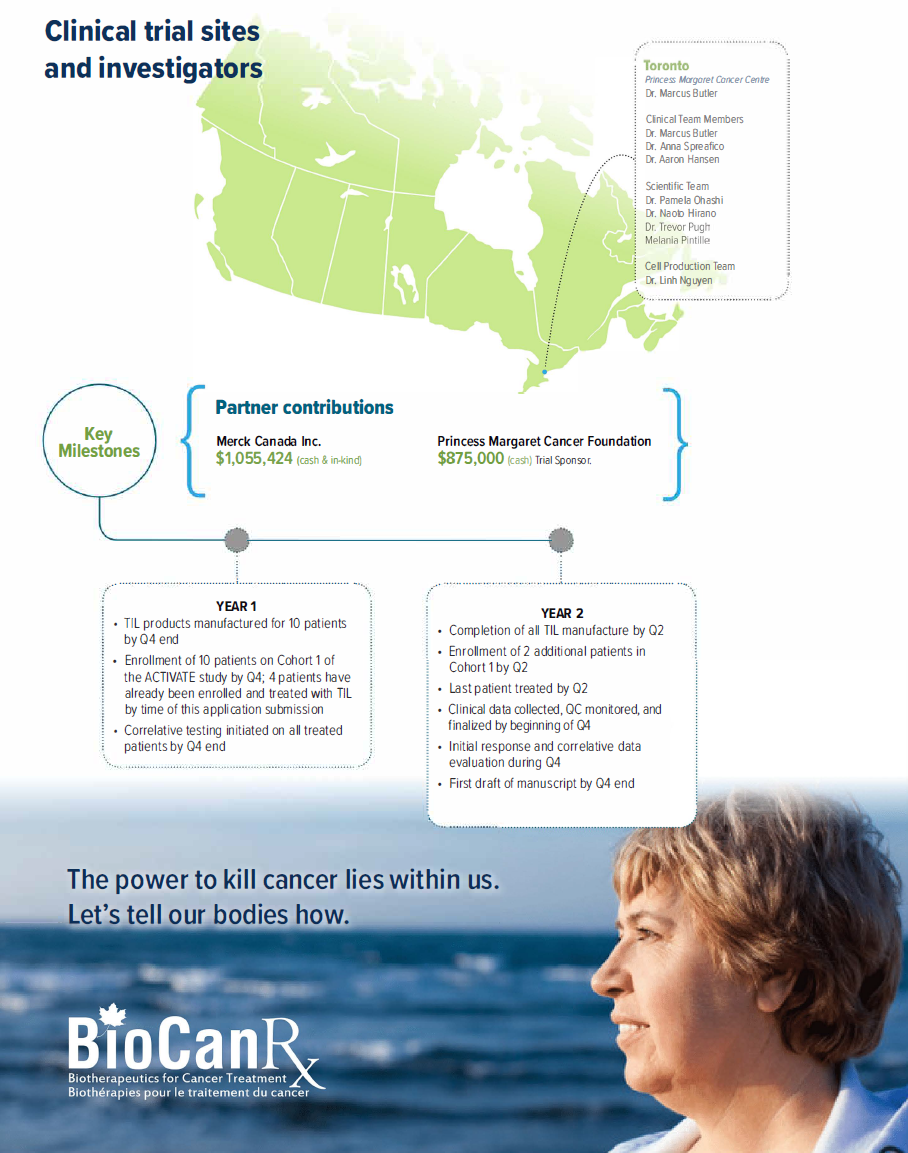
Project summary: Clinical Trial
Phase Ib trial of pembrolizumab administered following adoptive cell therapy- A multiple cohort study; The ACTIVATE (Adoptive Cell Therapy InVigorated to Augment Tumor Eradication) Trial.
April 23rd, 2018 to January 30, 2023
HIGHLIGHTS

- This therapeutic approach uses immune checkpoint inhibitors, a novel class of anti-cancer drugs and adoptive cell transfer (ACT) to eliminate cancer cells
- A number of preclinical studies suggest that a combination of checkpoint inhibitors and ACT could result in improved tumor control
- Pembrolizumab is a potent and highly selective humanized monoclonal antibody designed to directly block the interaction between PD-1 and its ligands PD-L1 and PD-L2
- Results from this study will determine the safety and effectiveness of the combination therapy and enable further development of cell therapies for Canadians suffering from different forms of cancer
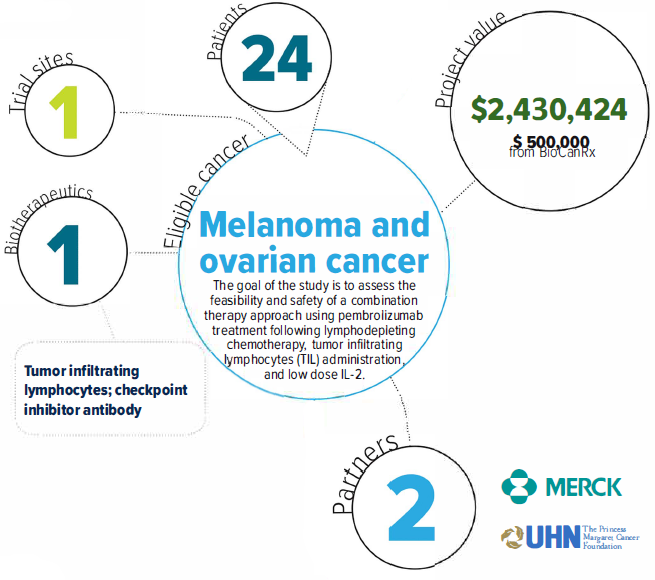
About the Project
The ACTIVATE clinical study will investigate the combination of adoptive cell transfer (ACT) and treatment with an immune checkpoint inhibitor in cancer patients for whom standard treatments have failed. ACT uses cancer-fighting immune cells grown in the laboratory to large numbers, then infused back to patients. Several Canadian groups, including the group led by Drs. Butler, Ohashi and Hirano at the Princess Margaret, are developing this strategy to bring better treatments to Canadians. This includes the use of tumor-infiltrating lymphocytes (TILs), immune cells that attack tumors. Long- lasting responses have been seen in some patients in clinical trials of TILs.
Immune checkpoint inhibitors are a new class of anti-cancer drug. Immune checkpoints are the “brakes” of the immune system, and sometimes cancer cells use these checkpoints to prevent immune cells from attacking the cancer. These drugs block this process, allowing immune cells to eliminate cancer cells. However, for most cancers, tumor shrinkage occurs in only a minority of patients after treatment. As with ACT, improvements are needed to benefit more patients. It has been observed in the laboratory and in some patients that combining checkpoint inhibitors with ACT shows the potential for improved responses. Given these promising results, we are performing a clinical study of the combination of TILs and the immune checkpoint inhibitor pembrolizumab. In this study, we are investigating the feasibility, safety and potential clinical benefit of this novel approach in cancer patients who are left with few therapeutic options.