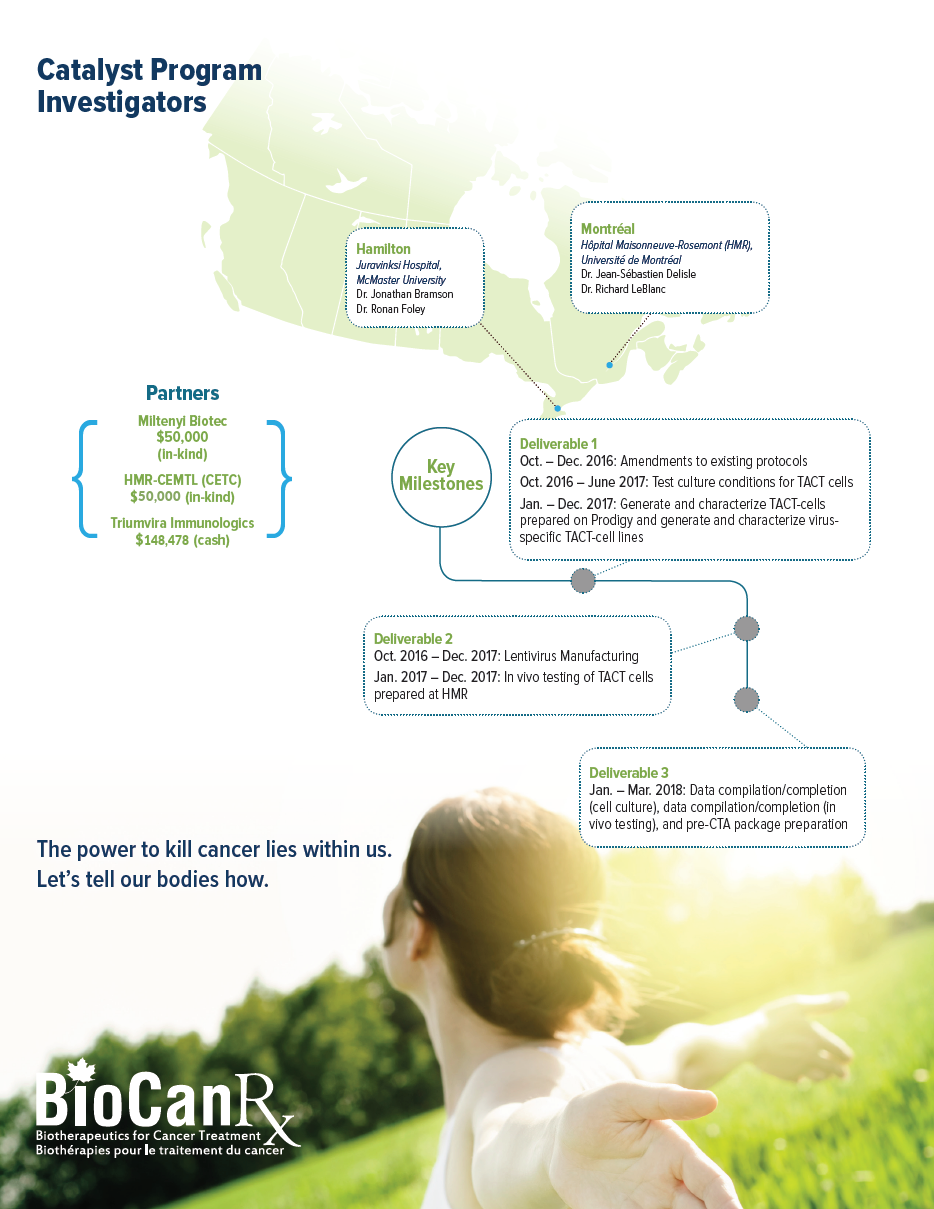
Project summary: Catalyst Program
Validation and manufacturing of anti-BCMA-TACT cells for the treatment of Multiple Myeloma
Oct 14, 2016 to Sept 30, 2018
HIGHLIGHTS

- TAC receptors are a novel Canadian technology with the potential to impact myeloma patient care worldwide and consolidate cell therapy manufacturing expertise in the country for other indications that involve gene modified T cell products
- Seeks to combine the novel technologies to yield a powerful, new treatment for multiple myeloma a clinical unmet need
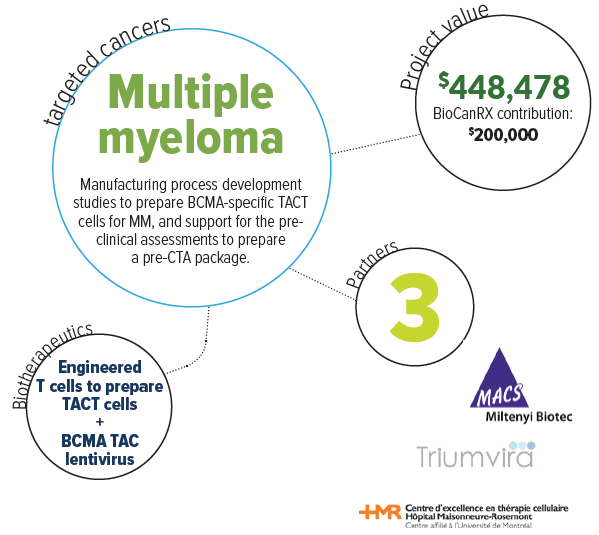
About this project
Multiple myeloma is recognized as an incurable illness despite significant improvements in the last 15 years. Mounting evidence suggests that immune mechanisms can cure this disease as well as many other blood cancers. Therapies based on immune cells that are modified in the laboratory and injected back into the patient have been found to be highly effective in the treatment of leukemia. Recent results have indicated that such modified immune cells may be useful for treating multiple myeloma.
The Bramson laboratory has developed a novel technology for producing immune cells that will attack multiple myeloma cells. The technology is a chimeric receptor, known as a TAC receptor that displays a distinct biology from the conventional chimeric antigen receptors (CARs) cells. The Delisle laboratory is developing novel methods to manufacture human immune cells known as T cells that can specifically target cancer cells and persist for a long time in the patient, thereby providing sustained anti-cancer effects. The manufacturing innovations tested in this project will be of value to T cell therapies. Successful completion of this project will increase the value of the Canadian-made TAC technology.
This project will combine the laboratories’ efforts to produce a powerful immune cell therapy for the treatment of multiple myeloma. At the end of the project period, the laboratories and clinical collaborators will be positioned with a clinical-grade manufacturing protocol that will support the development of a first-in-human clinical trial of this exciting new therapy.