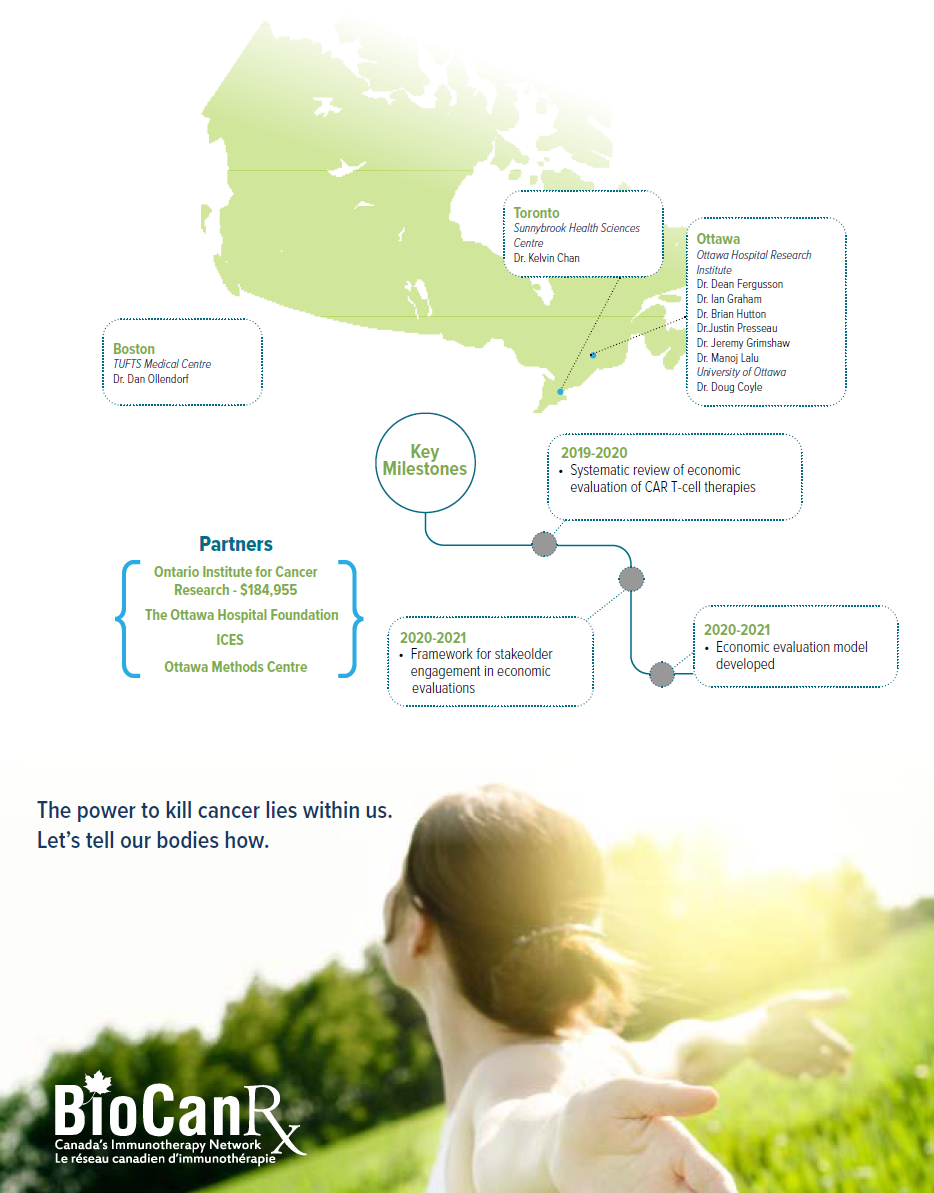
Project summary: Clinical, Social and Economic Impact Program
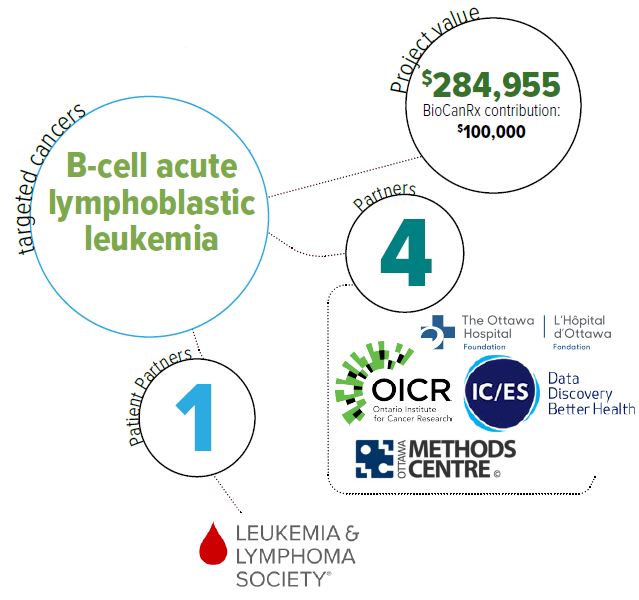
Using real-world data and iterative economic evaluation to prioritize resource allocation for care and research in adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia
April 1st, 2019 to June 30, 2022
HIGHLIGHTS

- Will assess the cost effectiveness of CAR T-cell therapy among adults with relapsed or refractory (r/r) B-cell ALL at an early stage of product development.
- Will assess whether it is feasible to integrate real world data, including Ontario’s health administrative and registry data to inform the economic evaluation of CAR T-cell therapies.
- Will develop a framework to engage stakeholders in economic evaluations.
About the Project
In the treatment of cancer, there are new therapies being discovered that use a cancer patient’s own immune cells to fight the cancer, generally known as immunotherapy. The therapy relies on a complicated manufacturing process that involves collecting a patient’s cells and then re-engineering them to attack cancer cells. This process can be long and costly, often having a high rate of failure to receive regulatory approval.
To better understand the factors contributing to the cost of this expensive treatment, the potential clinical benefits of the treatment, and what the health system can afford, we will apply a continuous economic evaluation framework to Chimeric Antigen Receptor (CAR) T-cell therapy that has just been approved in Canada for advanced leukemia but does not yet have a funding mechanism in Ontario.
Our process will actively involve multiple partners, including patients, CAR T developers, clinicians, researchers and healthcare payers. The results of the proposed study will help facilitate realistic commercial valuation of CAR T-cell therapy and assist public payers in setting a price of CAR T-cell therapy based on the additional benefit gained and the other associated costs of the treatment.