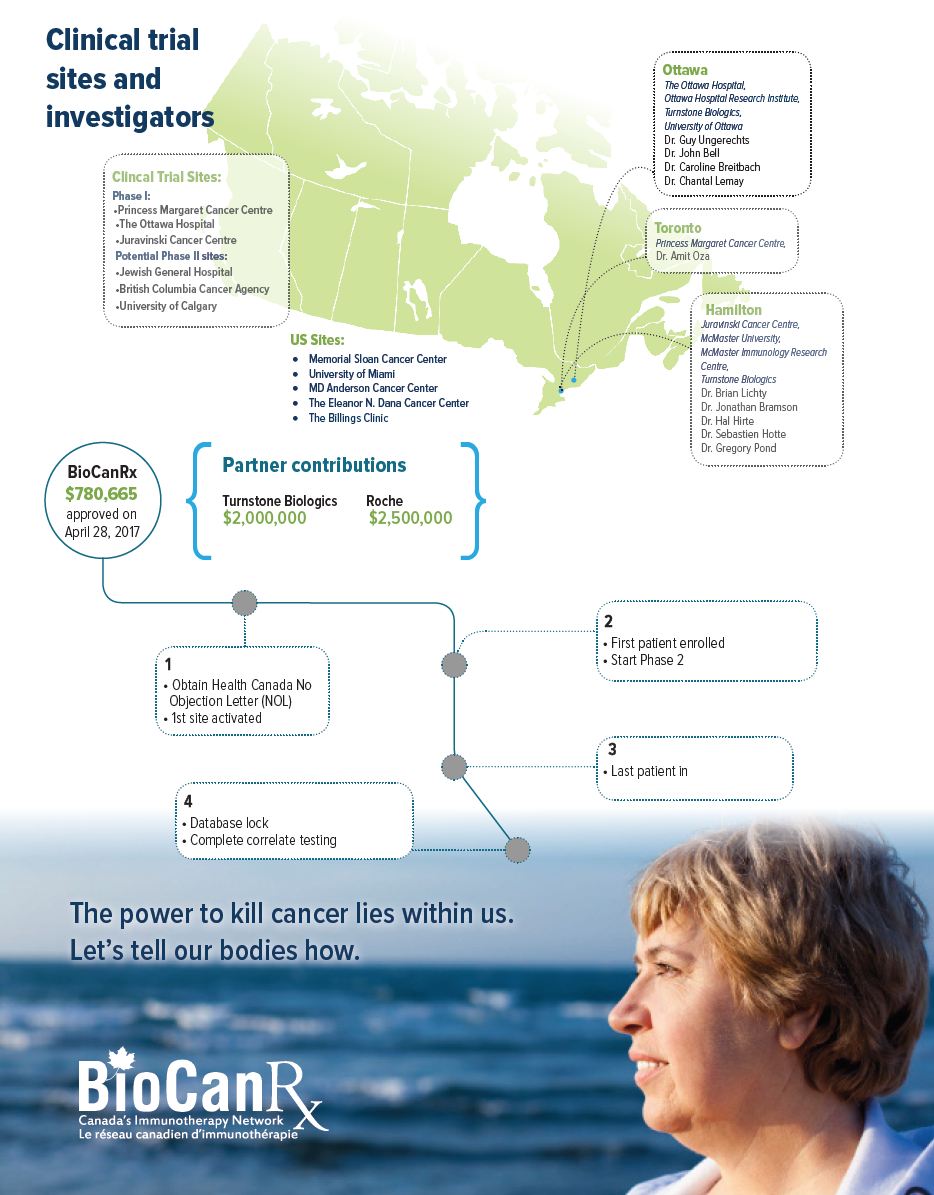
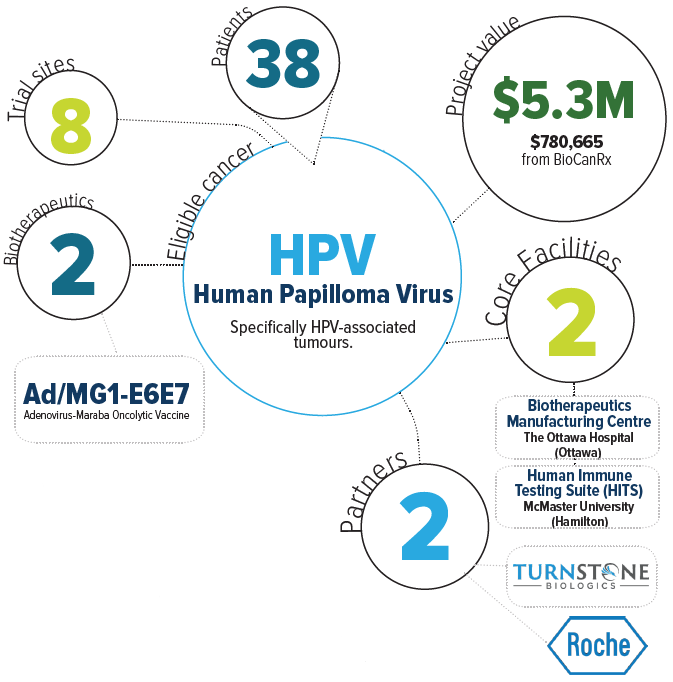
Project summary: Clinical Trials Program
A Phase 1/1b, Multicenter, Open-label Trial of Oncolytic MG1 Expressing Mutant Human Papilloma Virus (HPV) E6 and E7 (MG1-E6E7), with Adenovirus Vaccine Expressing Mutant HPV E6 and E7 (Ad-E6E7) and Atezolizumab in Patients with HPV Associated Cancers
July 1, 2017 to March 31, 2021
HIGHLIGHTS

- Evaluate the safety and anti-tumour activity of Ad/MG1-E6E7 in patients with HPV-associated tumours.
- Based upon oncolytic vaccine technology with a proven safety profile and unprecedented immune boosting activity.
- Open the trial at minimum of five Canadian sites.
About the project
Novel immunotherapy agents that target the immune system in order to treat tumours are revolutionizing cancer care. However, only 10-40% of patients respond to treatment, depending on the therapeutic agent and the indication. Therefore, novel agents with unique mechanisms-of-action are needed. Oncolytic virus vaccines infect and lyse tumour cells while inducing durable anti-tumour immunity by expression of tumour-associated antigens. This team of researchers has made a next-generation oncolytic virus vaccine that is designed to address a major unmet medical need: cancers associated with the human papilloma virus (HPV). HPV causes virtually all cervical cancers in the world, the majority of head and neck cancers in industrialized countries and a variety of other tumours (e.g. oesophageal and anal). Approximately 5% of the world’s cancer burden is caused by HPV.
HPV-associated tumours are an ideal indication for oncolytic vaccine technology as the HPV antigens are foreign (therefore highly immunogenic) and also drivers of tumourgenicity (therefore the tumour is not likely to lose expression of the antigen). This project aims to test in a clinical trial a new immune therapeutic strategy for patients with advanced cancers having failed conventional therapies. Specifically, the team have developed an oncolytic virus vaccine technology that takes advantage of the unique biology of a certain virus discovered by members of their team.
This project will evaluate the safety and anti-tumour activity of Ad/MG1-E6E7 in patients with HPV-associated tumours. In addition, anti-E6 and E7 immune responses will be monitored on the study, potentially providing a basis for the evaluation of the oncolytic virus vaccine technology in combination with other cancer immunotherapies in the future.
If clinical responses are observed on the proposed study, a pivotal study of Ad/MG1-E6E7 in an HPV-associated tumour indication is planned.