Project summary: Catalyst Program
CREATING T-CELL RECEPTORS THAT REACT TO SPECIFIC TUMOUR ANTIGENS FOR IMPROVED ADOPTIVE T-CELL THERAPY
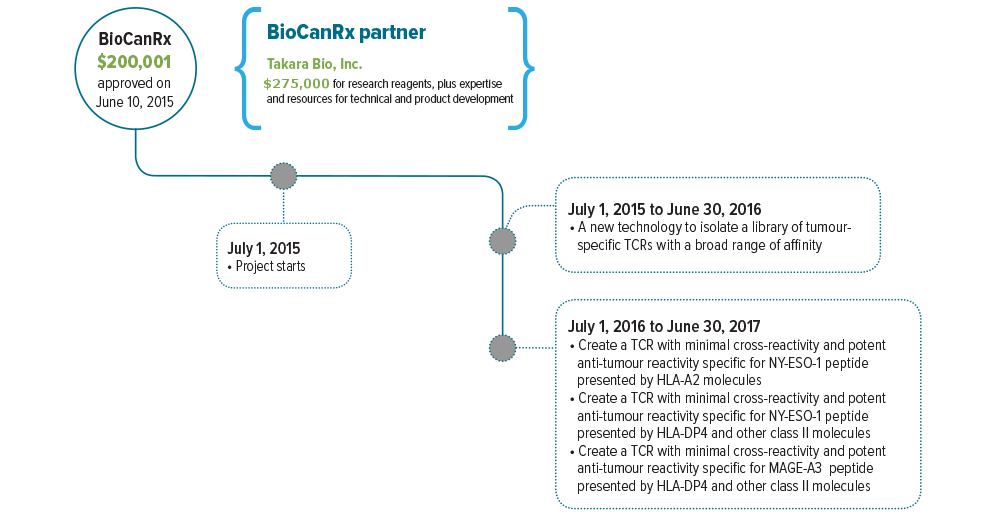
July 1, 2015 to June 30, 2017
HIGHLIGHTS

- Develops novel Canadian technology to improve anti-cancer effectiveness of T cells used for adoptive cell therapy
- Potential for more anti-tumour response with less toxicity for multiple forms of cancer
ABOUT THE PROJECT
Adoptive T-cell therapy is an emerging cancer immunotherapy that has shown great promise in recent early phase clinical trials. However, for any given cancer, only a small proportion of T cells within the tumour are actually programmed to recognize the cancer as a threat. To improve effectiveness of enlisting T cells in the fight against the tumour, there is a need to expand the population of T cells programmed to attack the targeted cancer, all without worsening side effects for patients.
Dr. Hirano’s lab has developed a technology to improve the quality of T cells by cloning T-cell receptors (TCRs) that are very sensitive to specific antigens found on a cancer, even more so than the T cells that naturally occur in a tumour. After creating these super cancer-sensitive TCRs, they are combined with T cells to create a fresh and active population of cancer-fighting T cells for delivery into a patient.
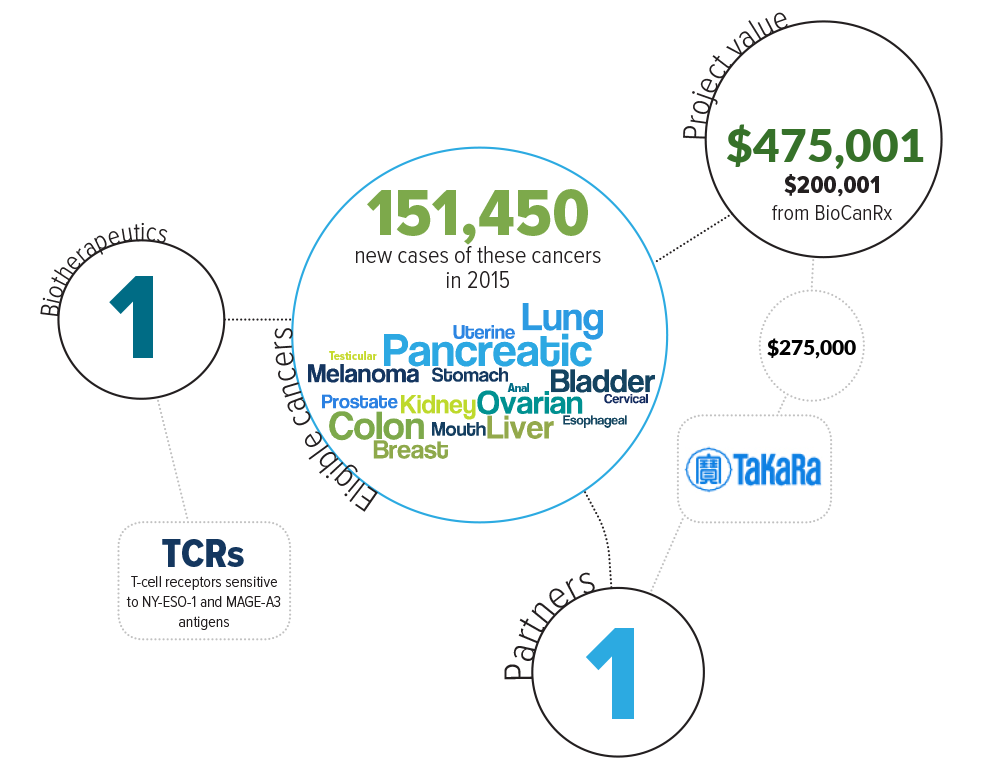
This project proposes to create TCRs that would target a wide range of cancer types. Specifically, Dr. Hirano’s group will create TCRs that are sensitive to the antigens NY-ESO-1 and MAGE-A3.
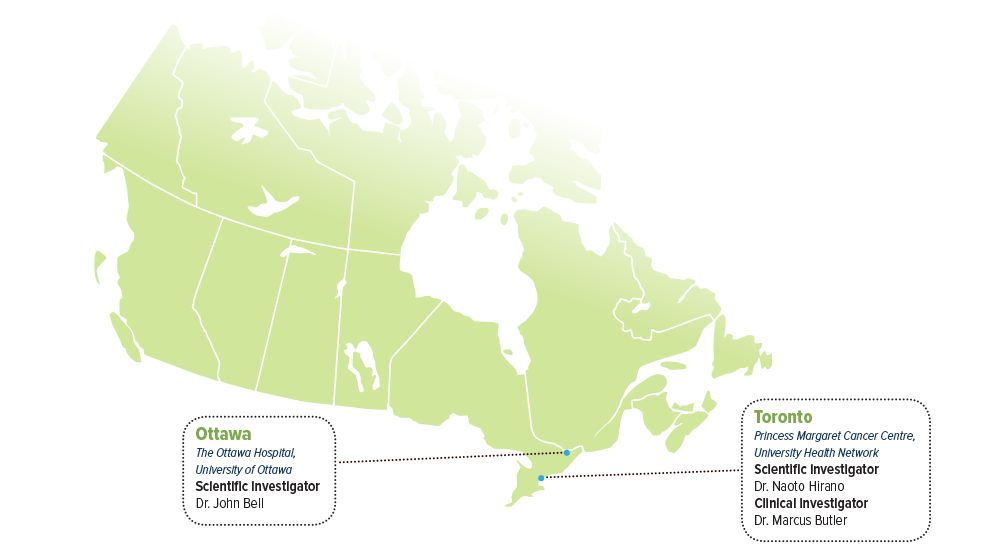
SCIENTIFIC INVESTIGATORS
- Dr. Naoto Hirano, Princess Margaret Cancer Center, University Health Network
- Dr. John Bell, The Ottawa Hospital, University of Ottawa
CLINICAL INVESTIGATOR
- Dr. Marcus Butler, Princess Margaret Cancer Center, University Health Network
Keywords: Adoptive T-cell therapy, T Cell receptors, TCR, MAGE-A3, NY-ESO-1
Eligible cancers: anal, bladder, breast, cervical, colon, esophageal, kidney, liver, lung, melanoma, mouth, ovarian, pancreatic, prostate, stomach, testicular, uterine
Partners: Takara Bio, Inc.

