Project summary: Catalyst Program
DEVELOPMENT OF AN ONCOLYTIC VACCINE FOR BRAIN CANCER
July 1, 2015 to Dec 31, 2016
HIGHLIGHTS

- Adapts the Canadian innovation of oncolytic vaccines to a potential treatment for glioblastoma multiforme (GBM)
- Evaluates two rhabdovirus platforms, Farmington (FMT) and Maraba, that are engineered to express the CMV antigens found in GBM tumours
- Builds on internationally recognized Canadian leadership in the development of oncolytic rhabdovirus vaccines
ABOUT THE PROJECT
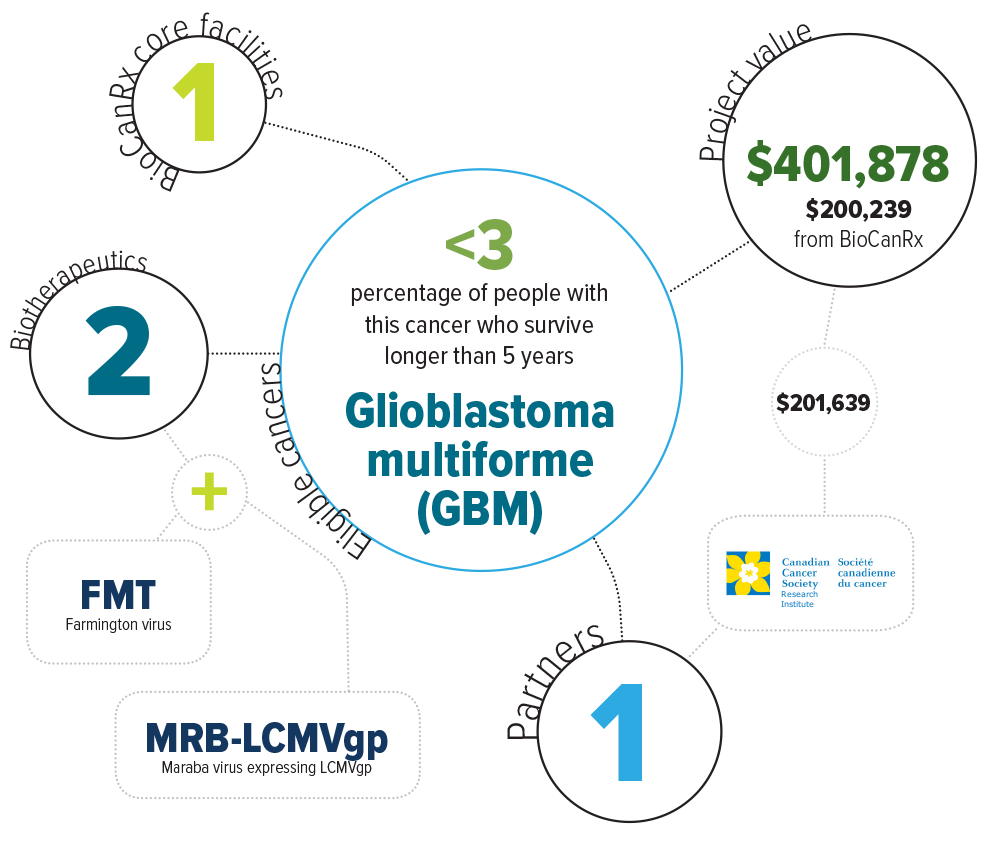
There have been no significant improvements in the treatment of glioblastoma multiforme (GBM) for the past 35 years. The overall five-year survival rate is less than 3% for GBM patients. Even for those who can undergo the current gold standard-of-care (surgical resection, radiation and the specialty chemotherapeutic, temozolomide) the five-year survival rate is just 34%. Clearly, there is a tremendous need to improve the outlook for this disease.
Dr. Stojdl’s lab has developed a new approach to GBM therapy that uses cancer-killing viruses to harness a patient’s own immune cells to fight their tumour. This immune activation is critically important during oncolytic virotherapy because patients whose tumours are packed with immune cells have a much better prognosis. These viruses have proven extremely safe in the brain and effective at dealing with issues that frustrate current GBM therapies.
This project will engineer an adapted virus designed to activate immune cell populations that are already established at high levels in the majority of individuals with GBM. Almost all GBM patients in Canada would be eligible for this therapy at the clinical trial phase. The virus will also be designed to act as a beacon that guides these activated immune cells to the tumour site.
With previous success in bringing oncolytic viruses to clinical trial, this streamlined and highly rational project is uniquely positioned to succeed in its goal of bringing this technology to Phase I/IIa trials, and ultimately vastly improving the outlook of GBM patients in Canada.
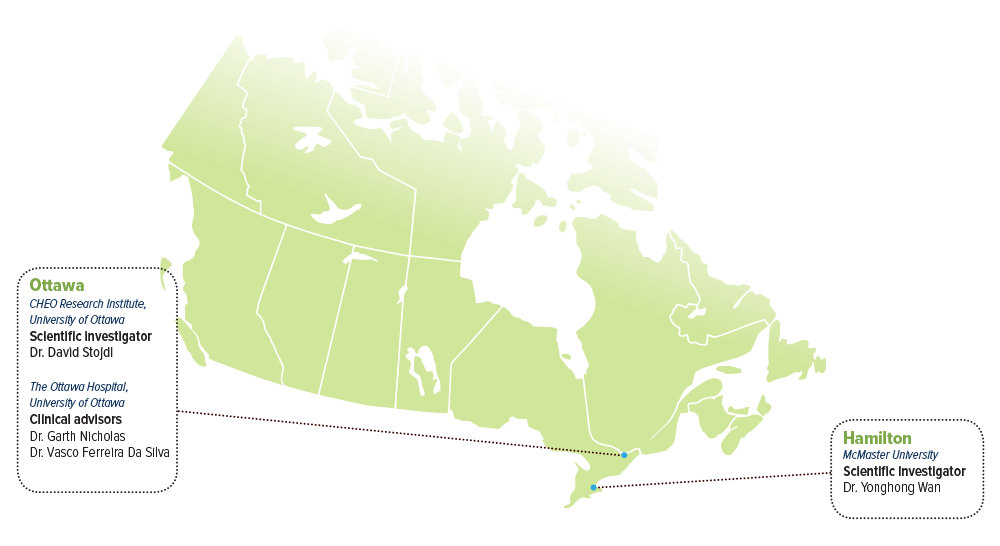
SCIENTIFIC INVESTIGATORS
- Dr. David Stojdl, CHEO Research Institute, University of Ottawa
- Dr. Yonghong Wan, McMaster University
CLINICAL ADVISORS
- Dr. Vasco Ferreira Da Silva, The Ottawa Hospital, University of Ottawa
- Dr. Garth Nicholas, The Ottawa Hospital, University of Ottawa
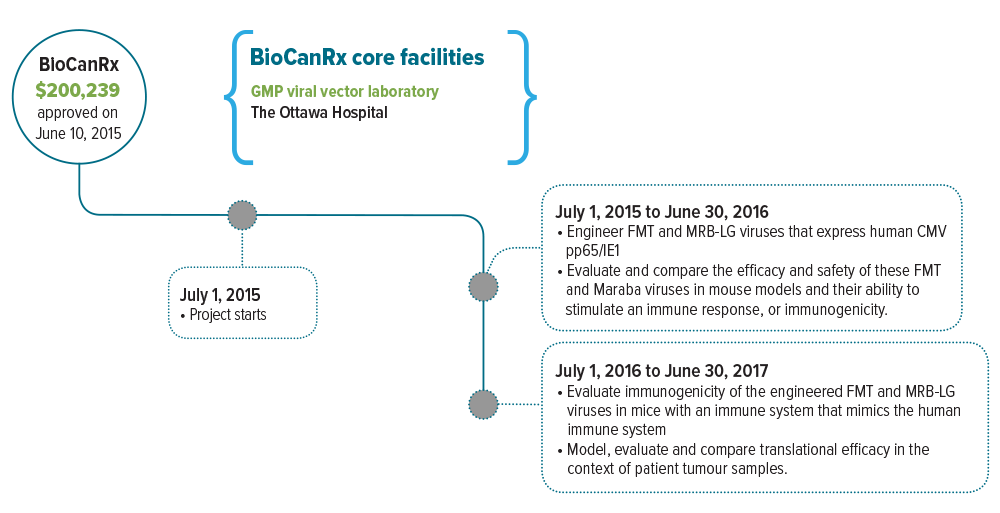
Keywords: oncolytic virus, oncolytic vaccine, Farmington virus, Maraba oncolytic virus, FMT, MRB-LCMVgp, CMV pp65/IE1 antigen
Eligible cancers: glioblastoma multiforme (GBM)
Partners: Canadian Cancer Society Research Institute

