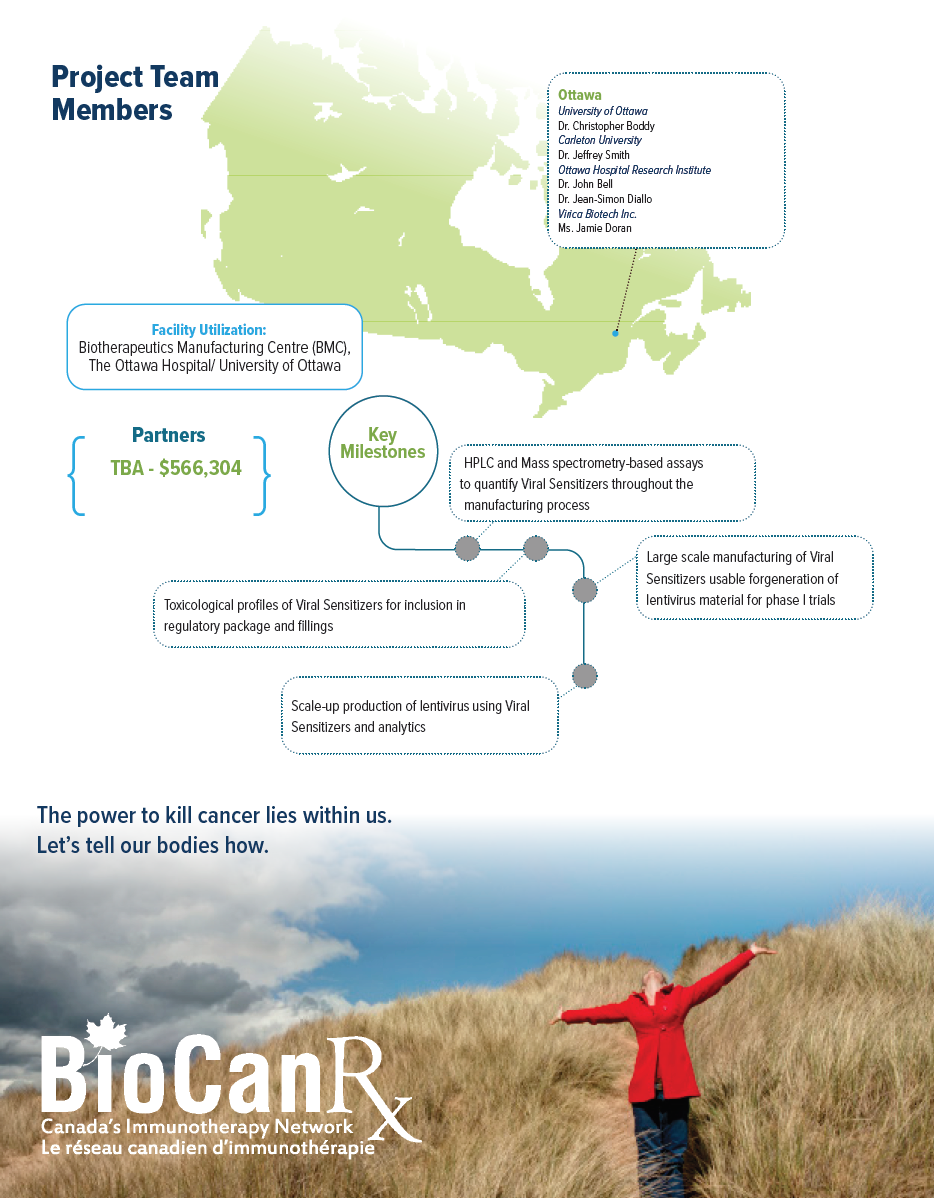
Project summary: Enabling Study
Development of Supporting Analytical Assays and Regulatory Compliance Package for Viral Sensitizer Technology Commercialization
July 1, 2020 – June 30, 2023
HIGHLIGHTS

- Lentivirus (LV)-engineered chimeric antigen receptor (CAR) T-cells have shown tremendous promise in clinical trials to treat hematopoietic cancers and constitute a flagship platform of the BioCanRx portfolio.
- The ability to produce sufficient virus to meet the demands of clinical evaluation and commercial launch is a key hurdle. In order to combat this, the Diallo team developed a scalable “Viral Sensitizer” formulation that enhances LV production.
- The researchers aim to develop a set of regulatory assays and procedures forming a critical path that will enable the advancement of this new technology for use by both academic institutes as well as large scale producers such as the BMC, one of BioCanRx’s core facilities which is manufacturing LV for ongoing Canadian CAR T trials.
About the Project
Oncolytic viruses and adoptive cell therapy are two highly promising anti-cancer strategies being developed by investigators within the BioCanRx network. The timely delivery of these therapeutics in sufficient quantities required for clinical testing relies on successful and efficient biomanufacturing of viral vectors, a process that is costly, time-consuming and challenging to implement on a large-scale.
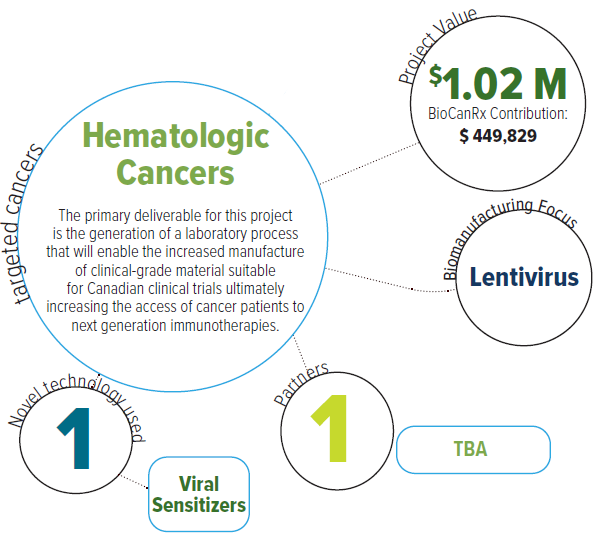
The researchers have developed a unique strategy using “viral sensitizers” (VSes), or a pool of bioactive small molecules formulated to robustly enhance the growth of viruses. Compared to other related technologies, these formulations are chemically defined and target antiviral defenses as opposed to cellular growth in order to boost viral production, in some cases upwards of 1,000-fold. This project aims to address the rising demand of efficient large-scale production of therapeutic viruses, notably lentiviral vectors for adoptive cell therapy.
Through support from a BioCanRx-funded Catalyst grant, a previous study has identified viral sensitizers formulations for small and large-scale lentivirus production. The researchers now aim to establish toxicology and stability profiles and to develop assays to track and quantify viral sensitizers and their metabolites for the implementation of this technology in biological manufacturing facilities.
Ultimately, this project will result in the generation of a laboratory process enabling the increased manufacture of clinical-grade material suitable for Canadian clinical trials ultimately increasing the access of cancer patients to next generation immunotherapies. These methodologies will also be employed to support the use of VSes for other purposes, such as the manufacturing of autologousinfected cell vaccines for other cell therapies.