News Release
CAR-T Project Dashboards
- Enabling Study Project - Capacity building for Chimeric Antigen Receptor (CAR)-modified T cell therapies in Canada (English only)
- Clinical, Social and Economic Impact Program - Getting better Outcomes with Chimeric Antigen Receptor T-cell therapy (GO–CART): A BioCanRx Research Excelerator to Safely and Effectively Translate CAR-T Cell Therapy for Hematological Malignancies (English only)
All Project Dashboards
Documents
Photos
- Dr. John Bell, Scientific Director, BioCanRx; Sr. Scientist, Centre for Innovative Cancer Research, Ottawa Hospital Research Institute and Professor, Departments of Medicine and Biochemistry, Microbiology & Immunology, University of Ottawa
- Dr. Robert Holt, distinguished scientist, BC Cancer Agency; Head of Sequencing and Head of Quality Systems, Canada’s Michael Smith Genome Sciences Centre; Professor, Medical Genetics, University of British Columbia and Professor, Molecular Biology and Biochemistry, Simon Fraser University
- Dr. Manoj Lalu, Associate Scientist, Assistant Professor, Clinical Epidemiology and Regenerative Medicine Programs, Ottawa Hospital Research Institute, The Ottawa Hospital and Department of Anesthesiology and Pain Medicine, University of Ottawa
- Dr. Natasha Kekre, Assistant Professor, Associate Scientist, Hematologist, Blood and Marrow Transplant Program, Ottawa Hospital Research Institute, The Ottawa Hospital, University of Ottawa.
- Stéphanie Michaud, PhD, President & Chief Executive Officer, BioCanRx
Graphics
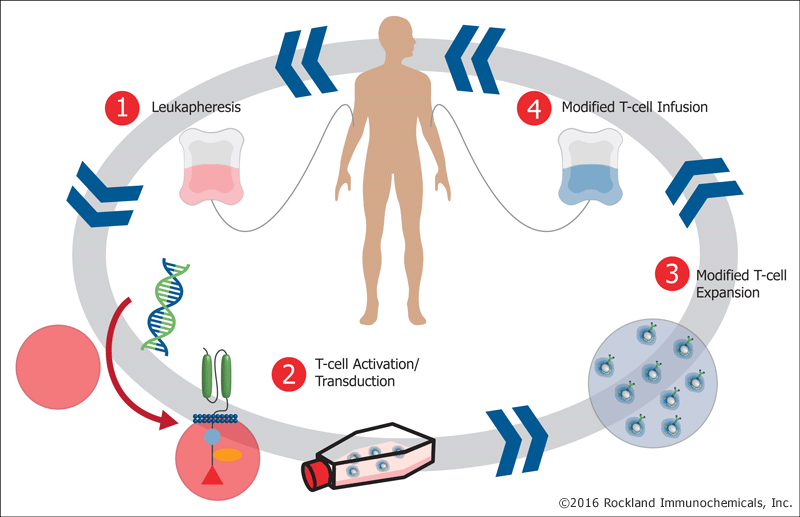
Image used with permission from Rockland Immunochemicals, Inc. (http://www.rockland-inc.com/)
A typical CAR T cell therapy involves four basic steps. (1) A patient or donor is leukapheresed to isolate peripheral blood mononuclear cells (PBMCs). (2) These cells are manipulated to express CAR by gene transfection. (3) CAR-expressing cells are differentiated into effector immune cells and expanded to sufficient number in vitro . (4) The CAR effector cells are then introduced into the patient. This is frequently done in conjunction with either chemo- or radio- therapy.
Videos
- Dr. John Bell on CAR-T therapy
- Dr. Manoj Lalu on CAR-T therapy clinical trial design
- B roll of Dr. John Bell's lab (1)
- B roll of Dr. John Bell’s lab (2)
- B roll of the Biotherapeutics Manufacturing Centre a GMP Viral Vector Core Facility, The Ottawa Hospital
- B roll of Process Development Suite, The Ottawa Hospital


