RESEARCH
Our Research Programs
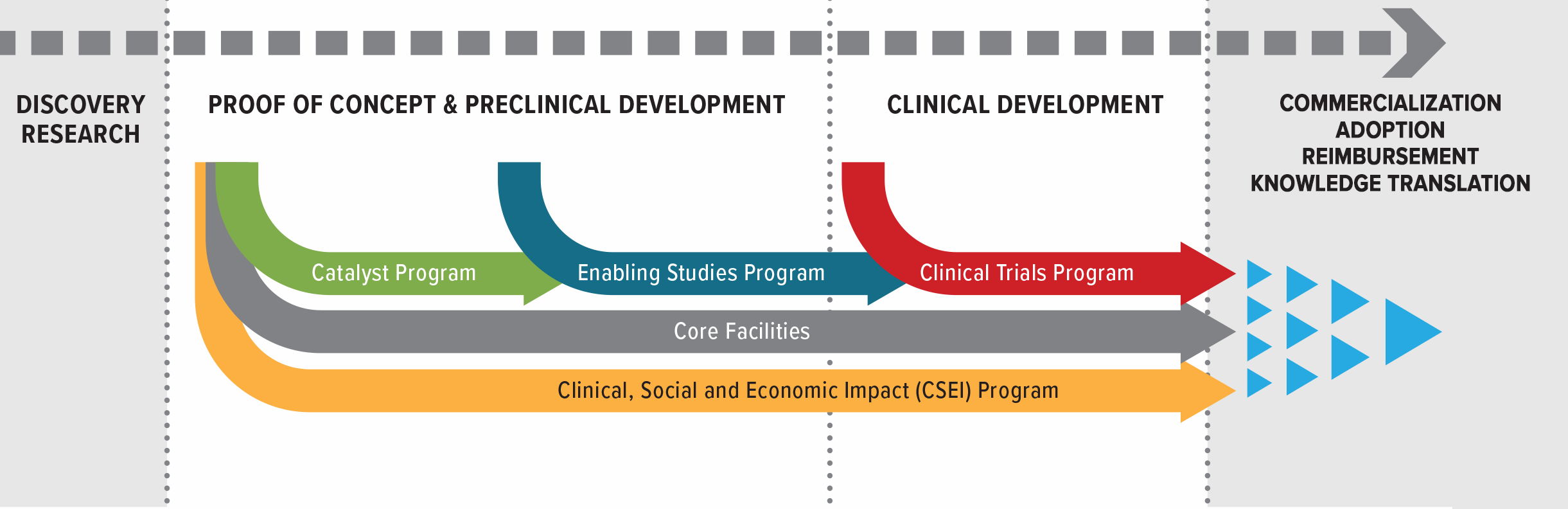
Research Programs
Accelerating discoveries from lab bench to patient bedside

Catalyst Program
The Catalyst Studies Program supports short-term, early-stage projects to conduct critical proof-of-concept studies that will advance cancer biotherapeutics development, assessment or clinical translation.
Program Highlights
- Projects are expected to be conducted over 6 months to 2 years
- BioCanRx funding budget request: $500,000
- BioCanRx will fund up to 50% of total project costs
- Catalyst Program projects are technology or product oriented; not fundamental science
Enabling Studies Program
The Enabling Studies Program funds work required to prepare and position biotherapeutic products and platforms for clinical testing in patients. It bridges the traditionally difficult-to-fund translation from the laboratory to clinical testing. Projects funded by the Enabling Studies Program are intended to provide critical resources to bridge the funding gap between laboratory discovery and clinical testing of innovations in cancer biotherapeutics, including funding to support GMP manufacturing and process development. Enabling Studies projects should result in either CTA submission packages or Quality (Chemistry and Manufacturing) packages.
Program Highlights
- Projects are expected to be conducted over 6 months to 3 years
- BioCanRx funding budget request: $750,000
- BioCanRx will fund up to 50% of total project costs
- Enabling Studies projects provide financial support and resources for activities required to position biotherapeutic products or platforms for their translation to clinical testing, including GMP manufacturing and process development, analytical assay development, and preclinical GLP studies.
- Enabling Studies projects must result in one or both deliverables: CTA submission packages and Quality (Chemistry and Manufacturing) packages
Clinical Trials Program
The Clinical Trials Program provides funds for Phase I/II clinical trials of novel cancer biotherapies that have been substantially developed in Canada. Clinical Trials projects are expected to be high-content, and academically driven. The program is focused on clinical trials using biotherapeutics, including but not limited to: oncolytic viruses and vaccines, cell therapies, and therapeutic antibodies, either as mono-therapies or in combination.
Program Highlights
- Projects are expected to be conducted over 1 to 3 years
- BioCanRx funding budget request: up to $1M total
- BioCanRx will fund up to 40% of the total project costs
- Clinical Trial projects must have novel, Canadian content in their approach
- The required deliverables include clinical data required to evaluate the case for advancing the therapeutic into later-stage clinical development.
Clinical, Social and Economic Impact (CSEI) Program
The objective of the Clinical, Social and Economic Impact (CSEI) Program is to develop potential solutions to social, legal, ethical, economic or health-systems barriers facing BioCanRx biotherapeutic products and platforms as they progress through the translational pipeline and reach patients in the evolving regulatory and policy landscape. The CSEI program funds projects that identify gaps and advance solutions in the uptake of BioCanRx cancer biotherapeutics and companion technologies by end users, which may include patients, researchers, healthcare providers, regulators, commercial partners, legal experts, payers, and policy makers. CSEI projects are expected to provide deliverables that assess economic, social, and/or commercial impact of cancer biotherapeutics, or inform decisions to advance therapeutics into later-stage clinical development/commercialization.
Program Highlights
- Projects are expected to be conducted over 1 to 3 years
- BioCanRx funding budget request: $215,000
- BioCanRx will fund up to 100% of total project costs
Core Facilities Program
Program Highlights
Continuing from the previous success of core facility funding, BioCanRx will provide funding for Canadian academic or research institution facilities that offer translational services. Funding from the Core Facilities Program is intended to provide a baseline level of support for core facilities that will be engaged in BioCanRx projects.
- BioCanRx funding for 2 years (with possibility of renewal for 3 additional years)
- BioCanRx funding budget request: up to $120,000 per year
Our Portfolio
Advancing Promising Therapies
Supports pre-clinical to Phase 1/2 trial
Funds proof of concept, dose & toxicity studies
Requires a clear path to the clinic
Facility & Impact Support
BioCanRx supports the infrastructure and activities that help our research have real-world impact. Funding core facilities and advancing technologies into practice, policy, and communities.
Core Facilities Support
We fund critical facilities involved in our projects when not covered by existing budgets.
- Facility staff and maintenance support
- Only when not already funded within project budgets
Advancing Technologies
We also provide funding to enable the forward path of these technologies.
- Clinical practice and program changes
- Tech adoption and dissemination