Our Model
Cancer Breakthroughs
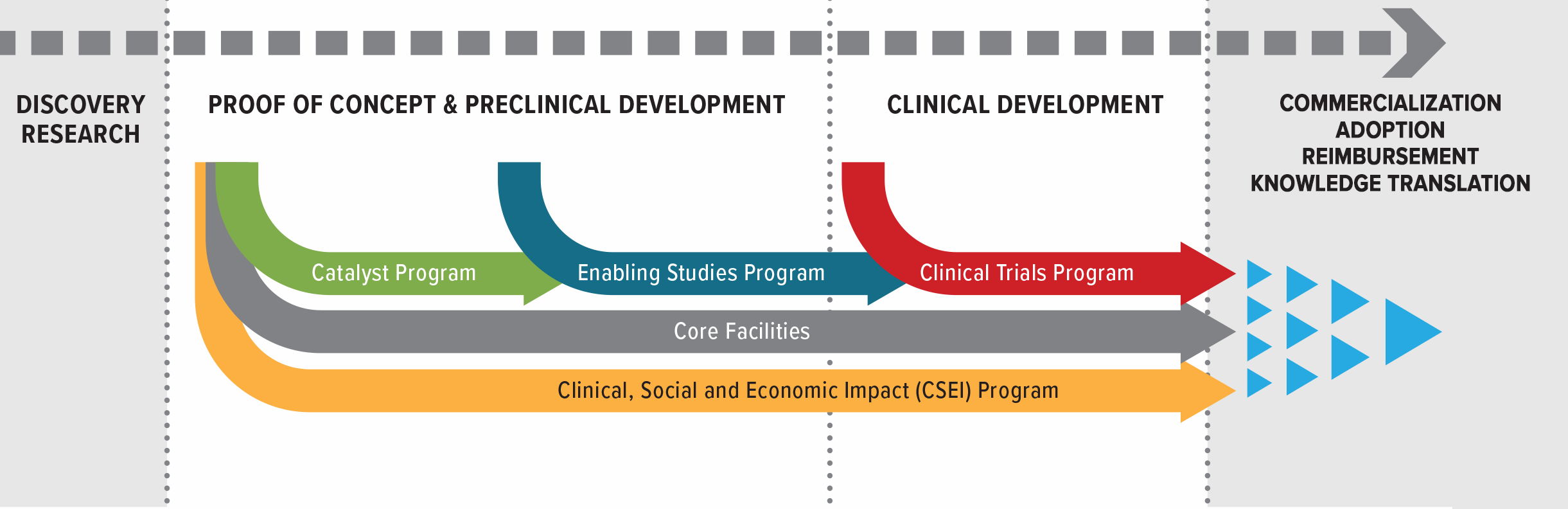
A Unique Approach in Canada
Our model invests in the most promising cancer immunotherapy research while training the next generation of scientists and connecting innovators with the expertise and infrastructure they need. By providing non-dilutive funding, we lower risk, increase value, and accelerate breakthroughs into the clinic.
Scalable across disease areas, this approach supports a future where personalized, precision medicine is accessible to all Canadians.
Investing in Breakthrough Research
Training the Next Generation
Connecting Innovators & Expertise
Focused Progress
Milestone-Driven Research
- If a therapy meets those milestones, it advances toward the clinic.
- If it does not, resources are redirected.
This ensures the efficient use of public funds, accelerates promising therapies, and avoids wasted effort on approaches that cannot deliver for patients.

Regulatory Alignment from the Start
Central to our model is building the evidence base required by Health Canada. By supporting projects to generate the right data early, we ensure therapies are positioned for real-world use and can move smoothly through the regulatory system. This alignment reduces delays, de-risks development, and increases the chances that innovations will reach patients.
Generate Data
Regulatory Alignment
Faster Approvals
Patients Benefit
Scalable Therapies
Built-In Manufacturing Readiness
Unlike traditional funding models, BioCanRx embeds Good Manufacturing Practice (GMP) biomanufacturing at the outset. This means therapies are:
- Scientifically robust
- Scalable and safe
- Ready for clinical application
This integration is critical for accelerating precision medicine, ensuring therapies are not only innovative but also manufacturable at a scale needed for a trial.

Our vision is bold yet clear: to transform cancer from a deadly disease into a curable one.
Our Impact
Integrated Planning with Patient Impact
Successful translation requires early integration of regulatory, manufacturing, and clinical planning. BioCanRx brings these elements together, always with patient outcomes as the north star.
Our model demonstrates how Canadian-made solutions, supported by a coordinated ecosystem, can move more efficiently from lab discovery to clinical trial — and ultimately, to patient benefit.