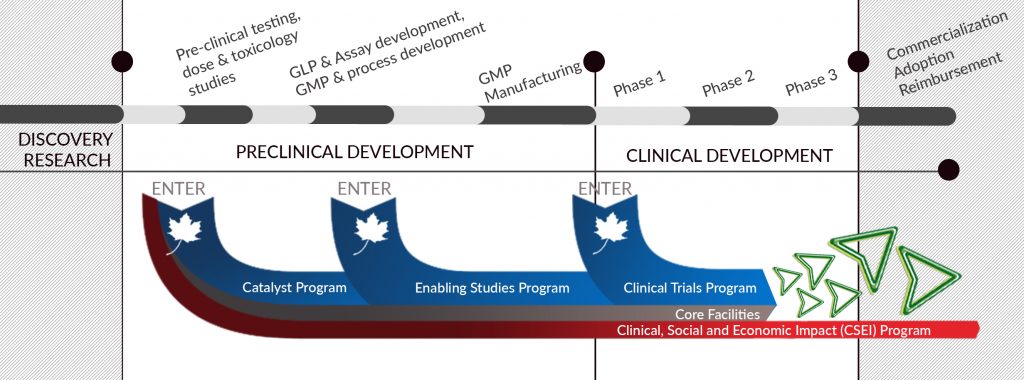
OUR PORTFOLIO
BioCanRx’s research programs support projects to transform lab ideas into clinical Phase 1 products. This means that projects can enter or exit the pipeline at different stages including pre-clinical models and testing, proof of concept, dose and toxicology studies and data collection. This also includes support for Phase 1/2 Clinical Trials. We do not invest in research that have not clearly identified a path to the clinic.
BioCanRx also provides funding support for essential core facilities that are engaged in our projects, which are not already receiving facility staff or maintenance support within the budgets of those BioCanRx projects. We also provide funding to enable the forward path of these technologies such as clinical practice and health decision making, dissemination and adoption, evidence-informed changes in policy and programs, community engagement.
OUR RESEARCH PROGRAMS
CATALYST PROGRAM
The Catalyst Program supports short-term, early stage projects that can advance to the next stage in the BioCanRx research pipeline, or generate scientific tools and methods that can be used by other BioCanRx network researchers. The Catalyst program was offered in Cycle 1; 18 Catalyst projects were funded in Cycle 1 totalling a BioCanRx investment of $3,116,800.
Program Highlights:
- BioCanRx contributes up to 50% for the costs of a Catalyst Program project.
- These projects are from six to 24 months in length.
- Catalyst Program projects are technology or product oriented; not fundamental science
- They are collaborative (across sectors or institutions)
Our Research Management Committee reviewed Catalyst projects for:
- A project with clear and direct relevance to cancer biotherapeutics; not a contrived story
- Catalyst Program projects could include:
- validation of manufacturing process improvements or companion technologies that may enhance manufacturing or testing of BioCanRx products
- validation of technologies that may apply to clinical evaluation of BioCanRx products (e.g., imaging, biomarker validation, immunological monitoring)
- proof-of-concept preclinical studies validating the efficacy of combining biotherapeutics
- A budget and project plan that makes sense
We’re not currently accepting applications for the Catalyst Program.
Browse our Funded Catalyst Projects.
ENABLING STUDIES PROGRAM
The Enabling Studies Program funds work required to prepare and position biotherapeutic products and platforms for clinical testing in patients. It bridges the traditionally difficult-to-fund translation from the laboratory to clinical testing. Data collected in these projects enables completion of a clinical trials application. To date, BioCanRx has funded 13 Enabling Studies in Cycle 1 and 6 Enabling Studies in Cycle 2, with a total investment of $9,398,368.
Program Highlights:
- BioCanRx will up to 50% for the costs of Enabling Studies.
- These projects could support GMP (good manufacturing process) and GLP (good laboratory process) studies, dosing and toxicology studies, process development, assay development and preparation for a clinical trial application (CTA).
- Enabling Studies must be collaborative (across sectors or institutions).
- They must result in one or both of these deliverables:
- CTA submission packages
- Quality (Chemistry and Manufacturing) packages
Our Research Management Committee reviews Enabling Studies projects for:
- a project whose outcome is a trial that we would fund through the Clinical Trials Program
- critical experiments that will lead to a clinical trial application and bolster its chances of approval by Health Canada
- a budget and project plan that makes sense
We’re not currently accepting applications for the Enabling Studies Program.
Browse our Funded Enabling Studies.
CLINICAL TRIALS PROGRAM
The Clinical Trials Program provides funds for Phase I/II clinical trials of novel cancer biotherapies that have been substantially developed in Canada. To date, BioCanRx has funded 8 Clinical Trials in Cycle 1 and 4 Clinical Trials in Cycle 2, with a total investment of $7,930,092.
Program Highlights:
- BioCanRx will contribute up to 40% for the costs of a clinical trial.
- The trial must have Canadian content in its approach (not a “me too” trial or a big pharma trial in disguise).
- The project must be collaborative, such as inclusion of multiple sites so the therapeutic is more widely available across Canada or use core facilities at other BioCanRx network institutions
Our Research Management Committee reviews Clinical Trial studies for:
- outstanding science in an international context
- Canadian innovation that is an integral element of the trial’s approach
- evidence that partnerships and funding will follow investment by BioCanRx
- therapies with a reasonable expectation that the health-care system will pay for them if a trial succeeds at Phase III
- a budget and project plan that makes sense
We’re not currently accepting applications for the Clinical Trials Program.
Browse our Funded Clinical Trials.
CLINICAL, SOCIAL AND ECONOMIC IMPACT (CSEI) PROGRAM
The objective of the CSEI Program is to develop potential solutions to social, legal, ethical, economic or health-systems barriers facing BioCanRx biotherapeutic products and platforms as they progress through the translational pipeline from preclinical research to clinical trials. To date, BioCanRx has funded 5 CSEI projects in Cycle 1, and 4 CSEI projects in Cycle 2 -for a combined investment of $2,203,318.
Program Highlights:
- BioCanRx will contribute up to 50% for the costs of a CSEI Program project
- CSEI Program projects must be collaborative (across sectors or institutions)
Our Research Management Committee reviews CSEI projects for:
- relevance to the BioCanRx mandate
- relevance to barriers in the translation of BioCanRx technologies into clinical testing and/or uptake by relevant receptors
- scientific excellence, creativity and innovation, in an international context
- feasibility of the study and of the access to required resources and expertise
- a team and project that is multidisciplinary and collaborative
- a commitment and level of involvement from partners, or potential for new partners to engage
- a budget and project plan that makes sense
We’re not currently accepting applications for the CSEI Program.
Browse our Funded CSEI Projects.
CORE FACILITIES PROGRAM
Our focus for this program has expanded in Cycle 2 to include not only baseline support for core facilities engaged in BioCanRx-funded projects, but also support that will establish additional, much needed biomanufacturing capacity in Canada.
Our traditional Core Program (Core Facilities) will continue to support the salary of HQP, minor expenses related to ongoing facility maintenance, certification and/or baseline operation for a maximum funding allocation of $100,000 per facility annually. BioCanRx-funded Core Facilities are selected based on:
- relevance to the BioCanRx mandate (i.e., accelerating translation of novel innovations in cancer biotherapeutics);
- extent of involvement in BioCanRx sponsored projects
- appropriate budget justification; and
- track record of the applicants/core facility.
Recognizing the critical need for increased access to biomanufacturing, and building off the success of our Core Program in Cycle 1, we developed an expanded program (Biomanufacturing Facilities) for Cycle 2 that will focus its support on bringing new facilities with sought after expertise online, onboarding new technology and facilitating knowledge transfer among facilities in an effort to provide affordable and sustainable access to biomanufacturing in Canada. Our Biomanufacturing Facilities program will focus on developing capacity in both vector and cell manufacturing, and will be an integrated and collaborative approach to GMP manufacturing with an emphasis on HQP training.
We’re not currently accepting applications for Core Facilities or Biomanufacturing Facilities. Browse our funded Core and Biomanufacturing Facilities.