Project summary: Enabling Studies Program
COMBINING ONCOLYTIC VACCINE THERAPY WITH ADOPTIVE CELL THERAPY TO TARGET CANCERS EXPRESSING MAGE-A3
July 1, 2015 to June 30, 2017
HIGHLIGHTS

- Enables application for a world-first clinical trial combining adoptive cell therapy with the internationally recognized Canadian innovation of oncolytic vaccines.
- Prepares an exciting combination of technologies that have a clear mechanism for working together to kill cancer cells.
ABOUT THE PROJECT
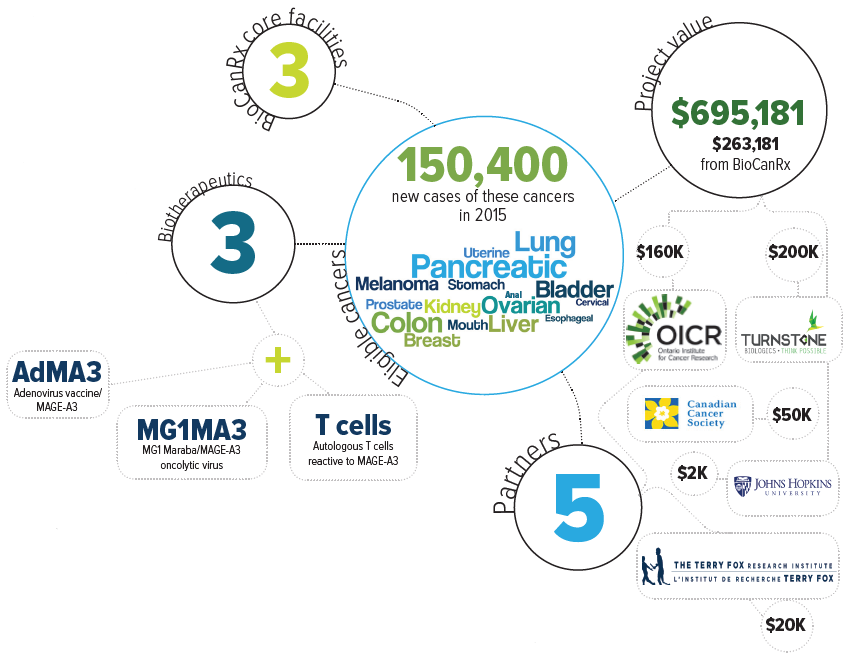
The goal of this project is to enable a potential combination therapy that capitalizes on two things: the recent U.S. successes of adoptive cell therapy (ACT) in early clinical trials; and the Canadian innovation of oncolytic vaccines. Oncolytic vaccine therapy makes the cancer visible to the immune system while also directly infecting and killing cancer cells.
The proposed oncolytic vaccine uses a genetically altered adenovirus (common cold) that primes the immune system to recognize a specific antigen found in many solid tumours — MAGE-A3. This is followed by the genetically altered oncolytic Maraba virus, which is engineered to also express MAGE-A3. In combination, these viruses enable a certain type of white blood cell, called T cells, to proliferate and see the cancer cells as foreign. The effect is an attack on the cancer by the T cells and by the Maraba virus. However, only a small group of the T cells produced in response to the oncolytic vaccine are the type that can attack cancer cells expressing MAGE-A3.
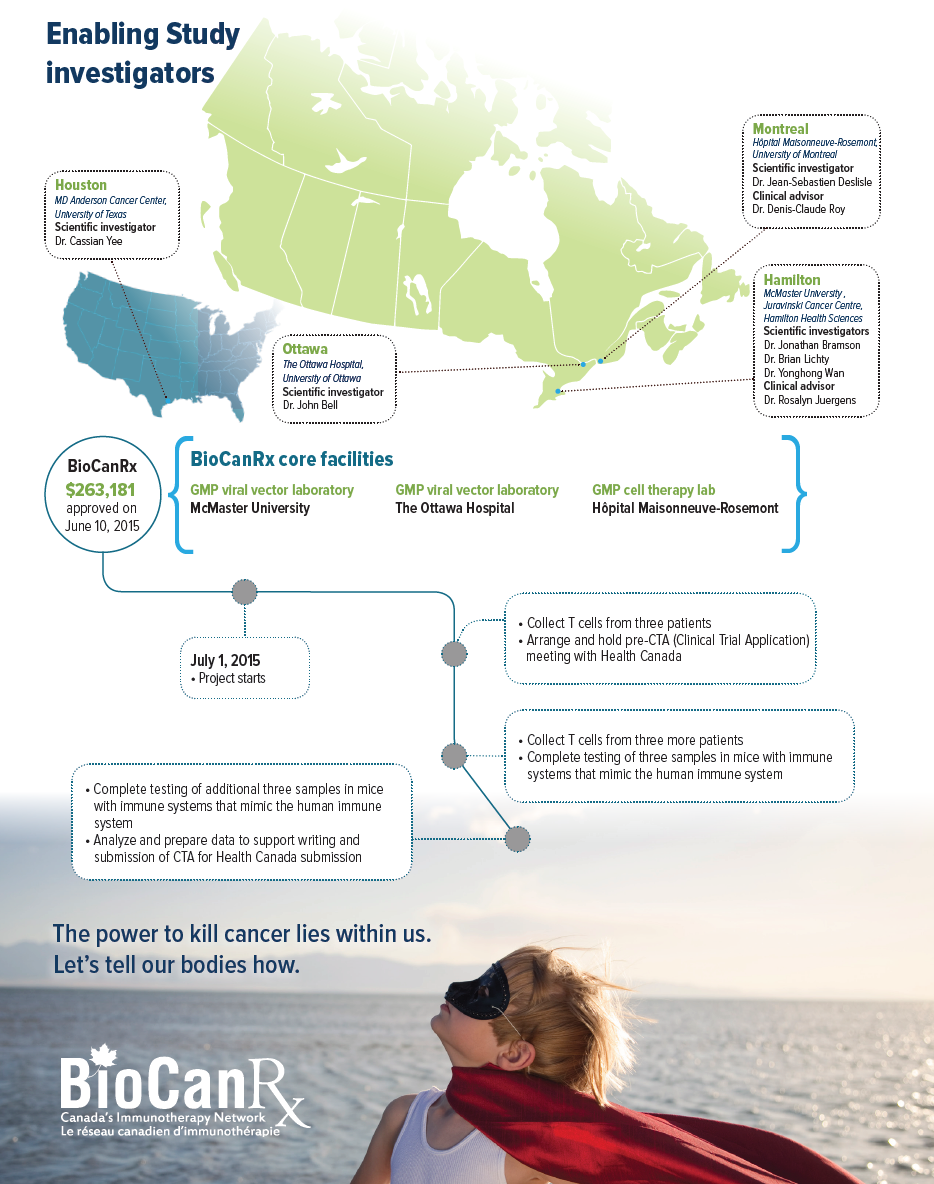
The project team proposes to supplement the oncolytic vaccine approach with T cells that have been programmed to search out the cancer cells expressing MAGE-A3. This would be an adaptation of a technology developed by Dr. Cassian Yee at MD Anderson Cancer Center in Texas using equipment not available in Canada. The funding will support the extraction of T cells from 6 patients in Canada that will then be sent to Texas for Dr. Yee to produce T cells programmed to see MAGE-A3. The activity of these cells will be measured before they are frozen and sent to back Canada, where potency tests will be performed. Should this be successful, the team in Montreal will adapt Dr. Yee’s method to the facilities available in Canada and compare the results.
SCIENTIFIC INVESTIGATORS
- Dr. John Bell, The Ottawa Hospital, University of Ottawa
- Dr. Jonathan Bramson, McMaster University, Juravinski Cancer Centre, Hamilton Health Sciences
- Dr. Jean-Sebastien Deslisle, Hôpital Maisonneuve-Rosemont, University of Montreal
- Dr. Brian Lichty, McMaster University, Juravinski Cancer Centre, Hamilton Health Sciences
- Dr. Yonghong Wan, McMaster University, Juravinski Cancer Centre, Hamilton Health Sciences
- Dr. Cassian Yee, MD Anderson Cancer Center, University of Texas
CLINICAL ADVISORS
- Dr. Rosalyn Juergens, McMaster University, Juravinski Cancer Centre, Hamilton Health Sciences
- Dr. Denis-Claude Roy, Hôpital Maisonneuve-Rosemont, University of Montreal
Keywords: oncolytic virus, oncolytic vaccine, adoptive cell therapy, autologous T cells, adenovirus vaccine, Maraba oncolytic virus, AdMA3, MG1MA3, MAGE-A3 antigen
Eligible cancers: anal, bladder, breast, cervical, colon, esophageal, kidney, liver, lung, melanoma, mouth, ovarian, pancreatic, prostate, stomach, uterine
Partners: Ontario Institute for Cancer Research, Turnstone Biologics

