Project summary: Enabling Studies Program
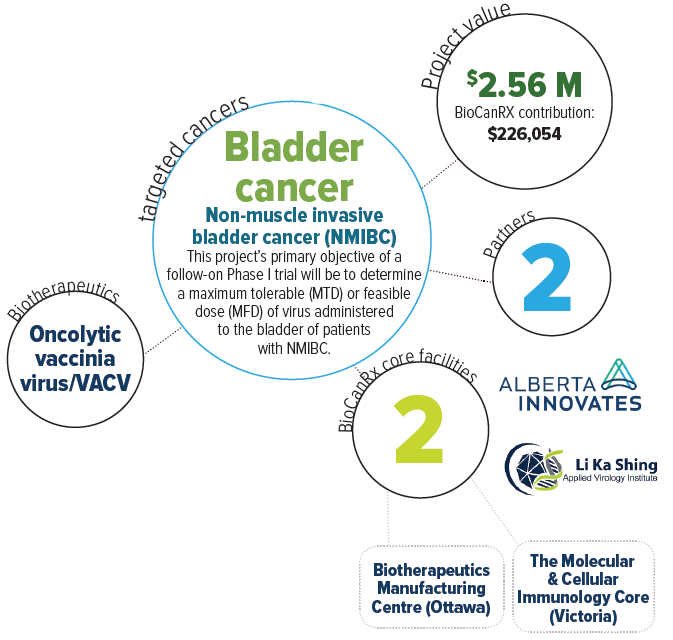
Oncolytic vaccinia virus for bladder cancer treatment: preparation for a phase I/II study
Jan 17, 2017 to Mar 31, 2021
HIGHLIGHTS

- An engineered strain of vaccinia virus (VACV) can address the need for more effective and curative BCa treatments
- Development work to enable the first trials that aim to provide new treatments to patients with early stage of NMIBC
- Early immunotherapy introduction might reduce the recurrence and progression of NMIBC to MIBC
About the project
Bladder Cancer (BCa) is the 5th most common cancer in Canada with a relapse rate of up to 80% and with many patients requiring multiple treatment strategies that often fail. Current treatments for BCa include surgery, chemotherapy, and/or intravesicular administration of Bacillus Calmette-Guerin (BCG) vaccine. While these may be initially effective, BCa has a high rate of recurrence thus requiring frequent and invasive monitoring. Furthermore, BCG can occasionally cause secondary mycobacterial infections and fails in 30% if patients. Recurrent BCa is often more aggressive and treatment resistant and at this stage treatment usually involves removing the bladder.
This research team believes that an oncolytic or “cancer-lysing” virus can address the need for more effective (and curative) BCa treatments. They have engineered a strain of vaccinia virus (VACV) to improve the safety and make it grow more selectively in cancer cells. They have shown that the virus can be administered via a catheter into the bladder of tumour-bearing animals and that the treatment is safe, selective for cancer calls, and causes both tumour regression and clearance. Furthermore, virus treatment promotes the development of an anti-tumour immune response, which prevents tumour recurrence, and is needed to generate sustained cures. The team are moving forward with the production of clinical grade virus. Their aim is to initiate a clinical trial following the completion of this project, with the goal of translating these exciting laboratory results into more effective treatments for patients afflicted with bladder cancer.
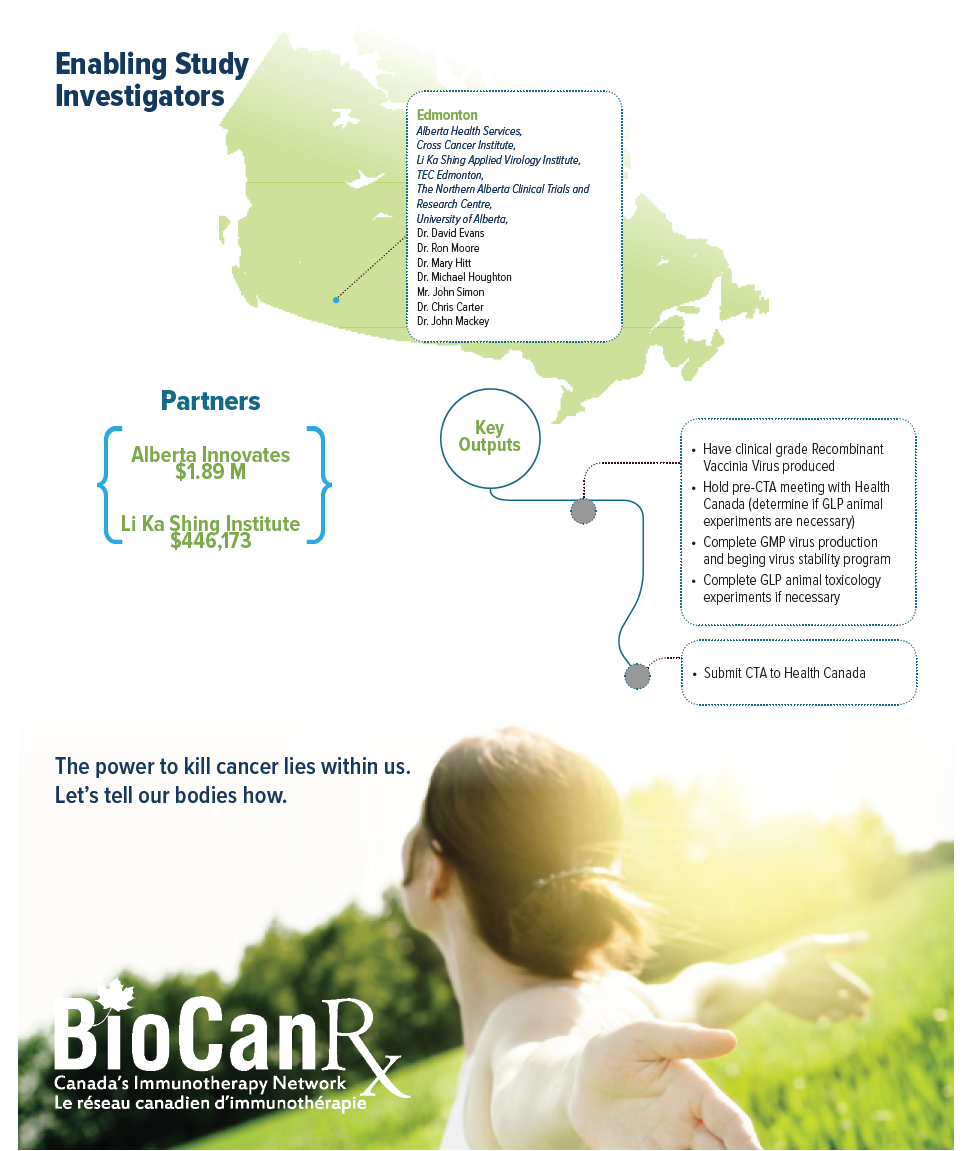
This team aims to start a phase I/II clinical trial in 2018-19. This will be performed at the University of Alberta, in conjunction with partners at Alberta Health Services, TECEdmonton, the Northern Alberta Clinical Trials & Research Centre (NACTRC), and the Cross Cancer Institute. Collectively, this project will develop a strong UA-based network capable of supporting the development of biologics for cancer therapy.