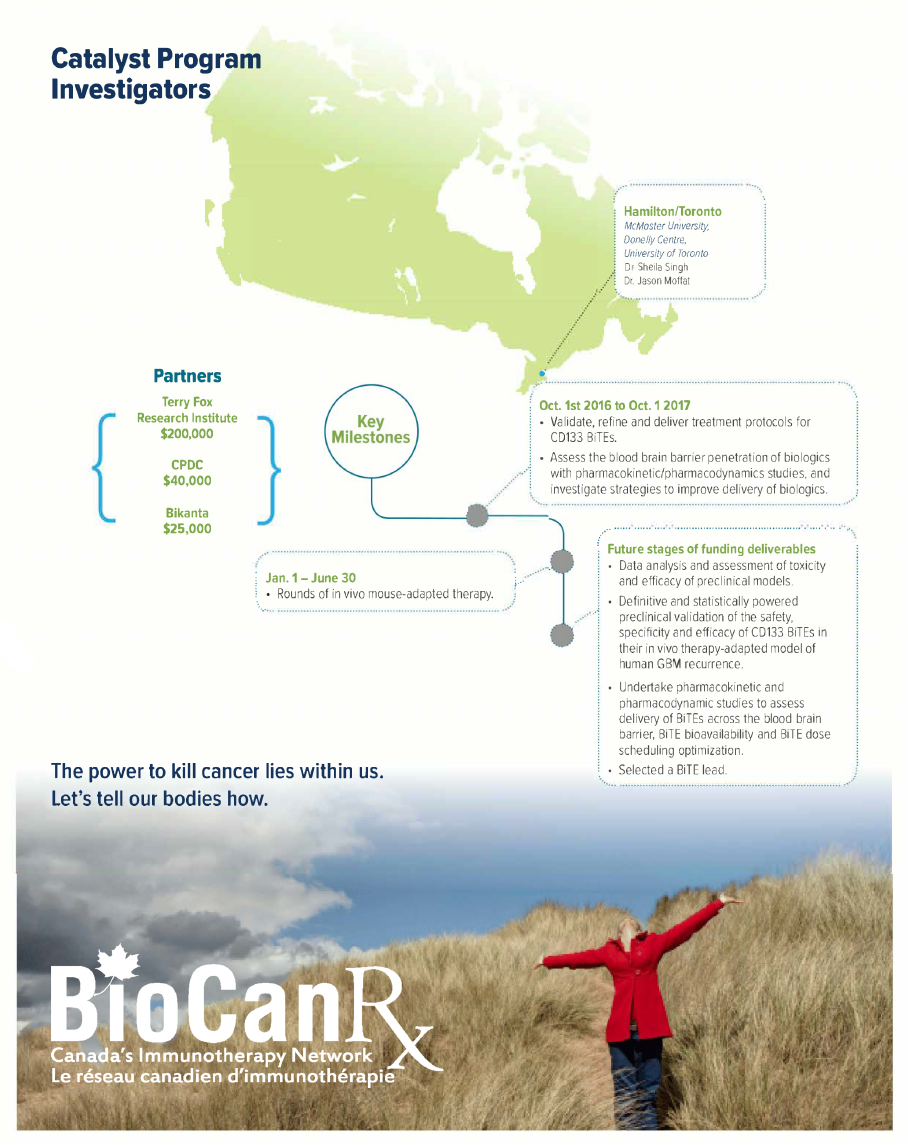
Project summary: Catalyst Program
Bispecific T-cell engager antibodies targeting CD133+ brain tumor-initiating cells: A novel immunotherapy for recurrent glioblastoma
Oct 14, 2016 to Sept 30, 2019
HIGHLIGHTS

- Development of a new promising therapeutic option for for future GBM patients using bispecific T-cell engager antibodies (BiTEs) to target cells that drive recurrent GBM
- Harnesses the immune system and directs T cells to specifically target CD133+ GBM cells
- Evaluates the development of custom-built BiTe designs for each TAA and epitope
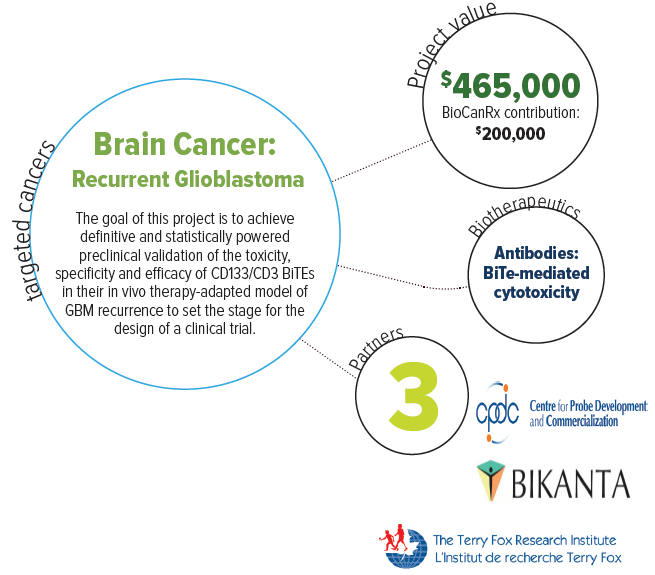
About this project
Glioblastoma (GBM) is the most common primary adult brain tumor and is typically highly aggressive, infiltrative and resistant to standard therapies. Even with surgery, standard chemotherapy with temozolomide (TMZ), and radiation, tumor re-growth (or recurrence) and patient relapse are inevitable. Patients typically face a <15 months survival rate, with fatal outcomes upon disease progression post-therapy. Clearly a new form of therapy is needed to help improve the outlook of this disease.
Recently a therapeutic model has emerged for GBM consisting of harnessing the patient’s immune system to attack the tumour. This paradigm can specifically kill those cancer cells by targeting the tumour’s proteins and drawing in the T cells of the immune system.
Dr. Singh and Dr. Moffat will combine their expertise in human GBM biology and clinically relevant patient-derived stem cell models of brain tumors, and human synthetic antibody engineering technologies, to develop effective immunotherapies to target recurrent GBM.
Their overall goal is to develop a therapeutic strategy targeting CD133 in recurrent glioblastoma, using newly designed and empirical immunotherapies that harness the immune system and direct T cells to specifically kill CD133+ GBM cells. They will also undertake preclinical evaluation of these novel therapeutic agents, called “BiTEs” or bispecific T-cell engager antibodies, using their unique animal model of human GBM recurrence.
After preclinical testing this project hopes to validate and translate its immunotherapeutic agent into early clinical development, generating targeted therapies and therapeutic benefits for future GBM patients.