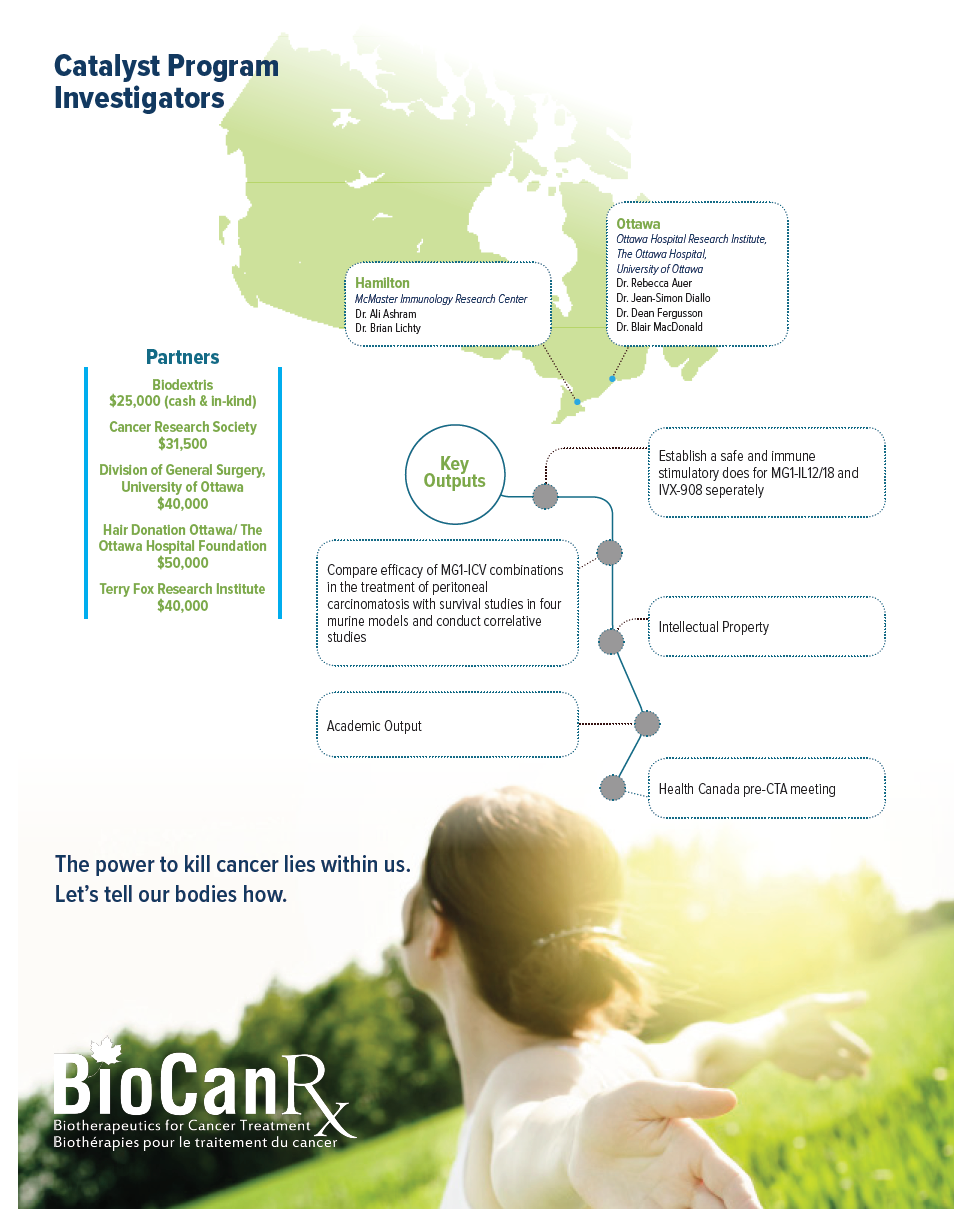
Project summary: Catalyst Program
Optimizing a personalized infected cell vaccine (ICV) for peritoneal carcinomatosis
Oct 14, 2016 to Sept 30, 2019
HIGHLIGHTS

- Preclinical models of colon cancer peritoneal carcinomatosis show that an ICV using the oncolytic virus Maraba expressing the immune stimulating protein, interleukin 12 (IL- 12), can eradicate multiple large tumours when delivered into the peritoneal cavity
- Evaluates the potentiating effects of IL18 and a TLR2/4 adjuvant on efficacy of an ICVs using an IL-12 expressing Maraba virus, the project’s lead clinical candidate
- Brings together a combination of clinical, methodological, scientific and commercial development expertise
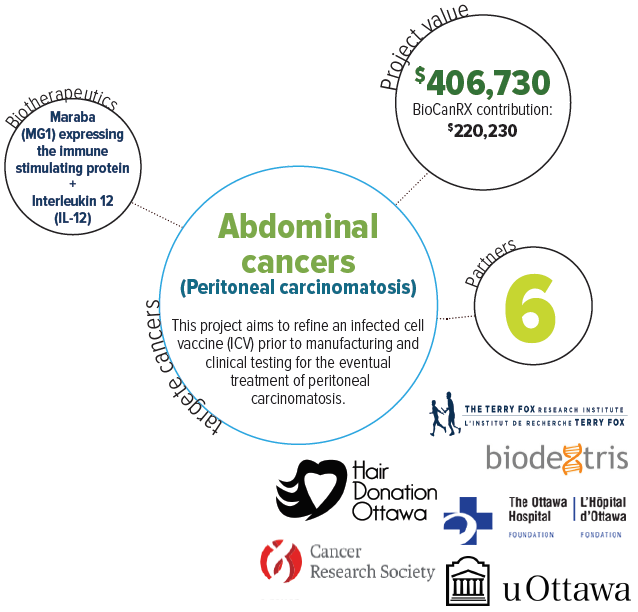
About this project
Peritoneal carcinomatosis (spread of cancer throughout the abdomen) is the leading cause of death for patients with abdominal cancers. Many patients die with massive abdominal distention, unable to eat or breathe comfortably. Despite the dismal prognosis, biotherapies hold significant promise, even in bulky and widespread disease. This study is proposing to optimize an infected cell vaccine (ICV) prior to manufacturing and clinical testing to address this pressing unmet clinical need.
A personalized ICV is made from an individual’s own tumour cells, harvested and infected with an oncolytic virus expressing an immune stimulatory protein. In preclinical models of colon cancer peritoneal carcinomatosis, they have demonstrated that an ICV using the oncolytic virus Maraba expressing the immune stimulating protein, interleukin 12 (IL-12), can eradicate multiple large tumours when delivered into the peritoneal cavity.
In collaboration with BioCanRx, and two Canadian start-up companies (Turnstone Biologics and Biodextris), they propose to further improve the efficacy of the ICV. At the end of the project an optimal ICV candidate will be identified to move forward with manufacturing and clinical trials.